Biochemistry Key Answers
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The Speed and Metabolic Cost of Digesting a Blood Meal Depends on Temperature in a Major Disease Vector Marshall D
© 2016. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2016) 219, 1893-1902 doi:10.1242/jeb.138669 RESEARCH ARTICLE The speed and metabolic cost of digesting a blood meal depends on temperature in a major disease vector Marshall D. McCue1,‡, Leigh Boardman2,*, Susana Clusella-Trullas3, Elsje Kleynhans2 and John S. Terblanche2 ABSTRACT and is believed to occur in all animals (reviewed in Jobling, 1983; The energetics of processing a meal is crucial for understanding McCue, 2006; Wang et al., 2006; Secor, 2009). Surprisingly, – energy budgets of animals in the wild. Given that digestion and its information on SDA among one of the largest taxonomic groups – associated costs may be dependent on environmental conditions, it is insects comes from very few studies (Table 1). Furthermore, the necessary to obtain a better understanding of these costs under SDA of insects remains poorly characterized in terms of the diverse conditions and identify resulting behavioural or physiological standard metrics used to characterize this phenomenon in other trade-offs. This study examines the speed and metabolic costs – in animals (e.g. magnitude, peak time, duration and coefficient). Given ’ cumulative, absolute and relative energetic terms – of processing a insects multiple roles as disease vectors, pests of agriculture, and as bloodmeal for a major zoonotic disease vector, the tsetse fly Glossina model taxa for evolutionary, climate and conservation-related brevipalpis, across a range of ecologically relevant temperatures (25, research, this constitutes a significant limitation for integrating 30 and 35°C). Respirometry showed that flies used less energy mechanistic understanding into population dynamics modelling, digesting meals faster at higher temperatures but that their starvation including population persistence and vulnerability to environmental tolerance was reduced, supporting the prediction that warmer change. -

Effect of Citric Acid Cycle Genetic Variants and Their Interactions With
cancers Article Effect of Citric Acid Cycle Genetic Variants and Their Interactions with Obesity, Physical Activity and Energy Intake on the Risk of Colorectal Cancer: Results from a Nested Case-Control Study in the UK Biobank Sooyoung Cho 1 , Nan Song 2,3 , Ji-Yeob Choi 2,4,5 and Aesun Shin 1,2,* 1 Department of Preventive Medicine, Seoul National University College of Medicine, Seoul 03080, Korea; [email protected] 2 Cancer Research Institute, Seoul National University, Seoul 03080, Korea; [email protected] (N.S.); [email protected] (J.-Y.C.) 3 Department of Epidemiology and Cancer Control, St. Jude Children’s Research Hospital, Memphis, TN 38105, USA 4 Department of Biomedical Sciences, Graduate School of Seoul National University, Seoul 03080, Korea 5 Medical Research Center, Institute of Health Policy and Management, Seoul National University, Seoul 03080, Korea * Correspondence: [email protected]; Tel.: +82-2-740-8331; Fax: +82-2-747-4830 Received: 18 August 2020; Accepted: 9 October 2020; Published: 12 October 2020 Simple Summary: The citric acid cycle has a central role in the cellular energy metabolism and biosynthesis of macromolecules in the mitochondrial matrix. We identified the single nucleotide polymorphisms (SNPs) of the citrate acid cycle with colorectal cancer susceptibility in UK population. Furthermore, we found the significant interaction of SNPs in the citric acid cycle with the contributors to energy balance and SNP-SNP interactions. Our findings provide clues to the etiology in cancer development related to energy metabolism and evidence on identification of the population at high risk of colorectal cancer. -

Nutrition and Metabolism
NUTRITION AND METABOLISM Metabolism - the sum of the chemical changes that occur in the cell and involve the breakdown (catabolism) and synthesis (anabolism) of stored energy sources. Basal Metabolic Rate is dened as the rate of energy production by the body measured under a dened set of conditions which is usually at rest (physical and mental), room temperature, 12 hours after a meal. The result is produced as a percentage of a standard value which is derived from studies of normal healthy people. Measurement of the metabolic rate takes place using a method called calorimetry. This may be done directly by measuring the amount of heat produced by the body in an Atwater chamber, the metabolic rate is the amount of heat produced per hour. More commonly the metabolic rate is determined indirectly by putting people on a closed circuit breathing system, with CO2 removed by a soda lime scrubber and the rate of oxygen consumption measured by change in volume. Oxygen consumption is proportional to the metabolic rate because most of the energy in the body is derived from oxidative phosphorylation, which uses a set amount of oxygen to produce a set amount of energy. For every litre of oxygen consumed the body produces (uses) 4.82 kcals of energy. If the oxygen consumption is 250ml/min (15L/hr) then the metabolic rate is 72.3 kcals/hr. This is often further rened by dividing the gure by the body surface area which for a 70kg male is 1.73m2. This gives an average BMR of approximately 40 kcal/m2/hr. -

Tricarboxylic Acid (TCA) Cycle Intermediates: Regulators of Immune Responses
life Review Tricarboxylic Acid (TCA) Cycle Intermediates: Regulators of Immune Responses Inseok Choi , Hyewon Son and Jea-Hyun Baek * School of Life Science, Handong Global University, Pohang, Gyeongbuk 37554, Korea; [email protected] (I.C.); [email protected] (H.S.) * Correspondence: [email protected]; Tel.: +82-54-260-1347 Abstract: The tricarboxylic acid cycle (TCA) is a series of chemical reactions used in aerobic organisms to generate energy via the oxidation of acetylcoenzyme A (CoA) derived from carbohydrates, fatty acids and proteins. In the eukaryotic system, the TCA cycle occurs completely in mitochondria, while the intermediates of the TCA cycle are retained inside mitochondria due to their polarity and hydrophilicity. Under cell stress conditions, mitochondria can become disrupted and release their contents, which act as danger signals in the cytosol. Of note, the TCA cycle intermediates may also leak from dysfunctioning mitochondria and regulate cellular processes. Increasing evidence shows that the metabolites of the TCA cycle are substantially involved in the regulation of immune responses. In this review, we aimed to provide a comprehensive systematic overview of the molecular mechanisms of each TCA cycle intermediate that may play key roles in regulating cellular immunity in cell stress and discuss its implication for immune activation and suppression. Keywords: Krebs cycle; tricarboxylic acid cycle; cellular immunity; immunometabolism 1. Introduction The tricarboxylic acid cycle (TCA, also known as the Krebs cycle or the citric acid Citation: Choi, I.; Son, H.; Baek, J.-H. Tricarboxylic Acid (TCA) Cycle cycle) is a series of chemical reactions used in aerobic organisms (pro- and eukaryotes) to Intermediates: Regulators of Immune generate energy via the oxidation of acetyl-coenzyme A (CoA) derived from carbohydrates, Responses. -

Citric Acid Cycle
CHEM464 / Medh, J.D. The Citric Acid Cycle Citric Acid Cycle: Central Role in Catabolism • Stage II of catabolism involves the conversion of carbohydrates, fats and aminoacids into acetylCoA • In aerobic organisms, citric acid cycle makes up the final stage of catabolism when acetyl CoA is completely oxidized to CO2. • Also called Krebs cycle or tricarboxylic acid (TCA) cycle. • It is a central integrative pathway that harvests chemical energy from biological fuel in the form of electrons in NADH and FADH2 (oxidation is loss of electrons). • NADH and FADH2 transfer electrons via the electron transport chain to final electron acceptor, O2, to form H2O. Entry of Pyruvate into the TCA cycle • Pyruvate is formed in the cytosol as a product of glycolysis • For entry into the TCA cycle, it has to be converted to Acetyl CoA. • Oxidation of pyruvate to acetyl CoA is catalyzed by the pyruvate dehydrogenase complex in the mitochondria • Mitochondria consist of inner and outer membranes and the matrix • Enzymes of the PDH complex and the TCA cycle (except succinate dehydrogenase) are in the matrix • Pyruvate translocase is an antiporter present in the inner mitochondrial membrane that allows entry of a molecule of pyruvate in exchange for a hydroxide ion. 1 CHEM464 / Medh, J.D. The Citric Acid Cycle The Pyruvate Dehydrogenase (PDH) complex • The PDH complex consists of 3 enzymes. They are: pyruvate dehydrogenase (E1), Dihydrolipoyl transacetylase (E2) and dihydrolipoyl dehydrogenase (E3). • It has 5 cofactors: CoASH, NAD+, lipoamide, TPP and FAD. CoASH and NAD+ participate stoichiometrically in the reaction, the other 3 cofactors have catalytic functions. -

Approved: Tre Specific Dynamic Action of Fat and P
TRE SPECIFIC DYNAMIC ACTION OF CARBOHYD.RATF, " FAT AND P.ROJ!EIN IN FIVE WOMEN by P.:l.tricia Jo ~~IG.n.'lley Thesis submitted to the Graduate faculty of the Virginie. Fblytechnic Institute in candidacy for the degree of MAmR OF SCIENCE in RUMAN NllfRITION AND FOODS APPROVED: "Ms.cy W. Korsliind • June, 1966 Blacksburg, Virginia -2- TABLE OF CONTENTS Page ·LIST OF TABLES • • • • • • • • • • • • • • • • • • • • • • 3 LIST OF FIGURES . " . 4 ACKNOWLEDGEMENT • • . .. 5 Chapter 1. INTRODUCTION . 6 II. REVIEW OF LITERATURE • . 9 Summary of Specific Dynamic Action Theories . 9 Specific Dynamic Action of Carbohydrate . • 10 Specific Dynamic Action of Fat , • . 14 Specific Dynamic Action of Protein . .. 15 III. METHODS AND PROCEDURES . 18 Subjects . 18 Adminilt~ation of Food and Measurements . .. 19 Collection and Analysis of Expired Air • . 20 Calculations . • • . • • • . .- . .. 20 IV. RESULTS AND DISCUSSION • • • • • • . .. 21 Changes in Respiratory Quotient Following the Ingestion of Carbohydrate, Fat and Protein •• . 21 V;irr-;iations in He11t Production After Ingestion of Carbohydrate, Fat and Protein • • • 28 V. SUMMARY •••••• . 37 BIBLIOGRAPHY • . 38 VITA •• . ... 44 APPENDIX . 45 TABLE NUMBlll PAGE 1. Time required, aft•l' the inge•tion of carbohydrate by f tve women, to reach highest re•piratory quotient and highest heat production. • • • • • • • • • • • • • • • • 31 2. Maximum increaee in heat production in. four women after ingestion of fat and time required for _.ximum increase to occur •••••••••••••••••• • • 31 3. Ma;xt... tacreaee in heat production in five women after ingeatioa of protein and time required for maximwll increaee to occur. • • • • • • • • • • • •. • • • • • • • 35 -4- LIST OF FIGURES FIGURE NUMBER PAGE 1~ Changes in reepiratory quotient in five women 22 after ingestion of sucrose. -

Specific Dynamic Action
Specific Dynamic Action ➢ The phenomenon of the extra heat production by the body, over and above the calculated caloric value, when a given food is metabolized by the body, is known as specific dynamic action (SDA). ➢ It is also known as calorigenic action or thermogenic action or thermic action (effect) of food. SDA for different foods ➢ For a food containing 25 g of protein, the heat production from the caloric value is 100 Cal (25 x 4 Cal). ➢ When 25 g protein is utilized by the body, 130 Cal of heat is liberated. ➢ The extra 30 Cal is the SDA of protein. ➢ SDA for protein, fat and carbohydrate 32%, 13% & 5%, ➢ Proteins possess the highest SDA while carbohydrates have the lowest. SDA for mixed diet ➢The presence of fats and carbohydrates reduces the SDA of proteins. ➢ Fats are most efficient in reducing SDA of foodstuffs. ➢ For a regularly consumed mixed diet, the SDA is around 10%. Significance of SDA ➢ For the utilization of foods by the body, certain amount of energy is consumed from the body stores. ➢ Expenditure by the body for the utilization of foodstuffs. ➢ It is the highest for proteins (30%) and lowest for carbohydrates (5%) & for mixed diet 10%. ➢ Additional 10% calories should be added to the total energy needs (of the body) towards SDA. ➢ The higher SDA for protein indicates that it is not a good source of energy. Mechanism of SDA ➢ SDA of foods is due to the energy required for digestion, absorption, transport, metabolism and storage of foods in the body. ➢ The SDA of proteins is primarily to meet the energy requirements for deamination, synthesis of urea, biosynthesis of proteins, synthesis of triacylglycerol (from carbon skeleton of amino acids). -
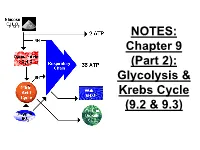
Chapter 9 (Part 2): Glycolysis & Krebs Cycle (9.2 & 9.3)
NOTES: Chapter 9 (Part 2): Glycolysis & Krebs Cycle (9.2 & 9.3) ● CELLULAR RESPIRATION: reactions in living cells in which sugars are broken down and energy is released Mitochondria in a Liver Cell!! Glucose + oxygen carbon dioxide + water + ENERGY C6H12O6 + 6O2 6CO2 + 6H2O + energy ● Food (glucose), like fuel, is “burned” by our cells for energy; however if it is burned all at once, too much energy is released. ● So, the reaction is broken down into many small steps controlled by ENZYMES ● the energy is transferred to the bonds of ATP which stores and releases the energy in usable amounts (packets) to be used by the cell Recall: the ATP cycle -Glucose = “large denomination” ($100) -ATP = “small change” ($1) *For each molecule of glucose, the cell can make approximately 36-38 ATP. Steps of Cellular Respiration: Phase of Occurs Starts Ends # of ATP Resp. where? with? with? made cyto- Glycoly- plasm 1 2 2 sis glucose pyruvate; NADH Krebs cycle (Citric Acid Cyc) E.T.C. (Resp Chain) & oxidative phosphor Steps of Cellular Respiration: Phase of Occurs Starts Ends # of ATP Resp. where? with? with? made cyto- Glycoly- plasm 1 2 2 sis glucose pyruvate; NADH Krebs inner 2 4 CO2, cycle matrix of pyruvate NADH, 2 (Citric Acid mito- FADH2 Cyc) chondria E.T.C. (Resp Chain) & oxidative phosphor Steps of Cellular Respiration: Phase of Occurs Starts Ends # of ATP Resp. where? with? with? made cyto- Glycoly- plasm 1 2 2 sis glucose pyruvate; NADH Krebs inner 2 4 CO2, cycle matrix of pyruvate NADH, 2 (Citric Acid mito- FADH2 Cyc) chondria E.T.C. -

Measurement of Individual and Population Energetics of Marine Mammals
Marine Mammal Ecology and Conservation / 08-Boyd-Ch08 page 165 7:55pm OUP CORRECTED PROOF – Finals, 5/7/2010, SPi 8 Measurement of individual and population energetics of marine mammals Sara J. Iverson, Carol E. Sparling, Terrie M. Williams, Shelley L.C. Lang, and W. Don Bowen 8.1 Introduction The overriding currency of all animal life is energy. Animals have evolved strategies of energy acquisition and use, but these strategies also experience tradeoffs between energy allocated to maintenance, activities, growth, and reproduction and are central to our understanding of life histories and fitness. Thus ‘energetics’— the study of the metabolic requirements, energy use, and output of animals— underpins many areas of physiology, ecology, evolutionary, and population biology, and even ecosystem dynamics. Marine mammals pose many challenges for the study, interpretation, and com- parison of individual and population energetics. For instance, the ability to study captive animals is often restricted to a few of the smaller marine mammal species. Although opportunities in the wild may be greater, there remain serious limits to our abilities to study species in remote locations (e.g. polar bears, Ursus maritimus,in the high arctic), in unstable habitats (e.g. ice-associated pinnipeds in the Bering and Chukchi Seas), or due to endangered status (e.g. monk seals, Monachus spp., southern sea otters, Enhydra lutris nereis, and vaquita, Phocoena sinus). As a group, cetaceans pose further difficulties because of their limited accessibility and large size. Adaptive insulation (blubber) is important in temperature management (Iverson 2009a) and the presence of this comparatively inert tissue can add complexity to the issue of defining the metabolically relevant body mass in marine mammals. -

Eႇects of Alternative Oxidase on Drosophila Under Environmental
6,1$6$$5, (ႇHFWVRI$OWHUQDWLYH 2[LGDVHRQDrosophila XQGHU(QYLURQPHQWDO6WUHVV 7DPSHUH8QLYHUVLW\'LVVHUWDWLRQV 7DPSHUH8QLYHUVLW\'LVVHUWDWLRQV 6,1$6$$5, (IIHFWVRI$OWHUQDWLYH 2[LGDVHRQDrosophila XQGHU(QYLURQPHQWDO6WUHVV $&$'(0,&',66(57$7,21 7REHSUHVHQWHGZLWKWKHSHUPLVVLRQRI WKH)DFXOW\RI0HGLFLQHDQG+HDOWK7HFKQRORJ\ RI7DPSHUH8QLYHUVLW\ IRUSXEOLFGLVFXVVLRQLQWKHDXGLWRULXP) RIWKH$UYREXLOGLQJ$UYR<OS|QNDWX7DPSHUH RQ2FWREHUDWR¶FORFN $&$'(0,&',66(57$7,21 7DPSHUH8QLYHUVLW\)DFXOW\RI0HGLFLQHDQG+HDOWK7HFKQRORJ\ )LQODQG Responsible 3URIHVVRU+RZDUG7-DFREV supervisor 7DPSHUH8QLYHUVLW\ and Custos )LQODQG Pre-examiners 3URIHVVRU:LOOLDP-%DOODUG 'RFHQW(LMD3LULQHQ 8QLYHUVLW\RI1HZ6RXWK:DOHV 8QLYHUVLW\RI+HOVLQNL $XVWUDOLD )LQODQG Opponent $VVRFLDWH3URIHVVRU9LOOH+LHWDNDQJDV 8QLYHUVLW\RI+HOVLQNL )LQODQG 7KHRULJLQDOLW\RIWKLVWKHVLVKDVEHHQFKHFNHGXVLQJWKH7XUQLWLQ2ULJLQDOLW\&KHFN VHUYLFH &RS\ULJKWDXWKRU &RYHUGHVLJQ5RLKX,QF ,6%1 SULQW ,6%1 SGI ,661 SULQW ,661 SGI KWWSXUQIL851,6%1 3XQD0XVWD2\±<OLRSLVWRSDLQR 7DPSHUH To Pulla iii iv ABSTRACT The mitochondrion is a unique organelle with a central role in energy production and metabolic homeostasis while regulating the life and death of the entire cell. In mitochondrial dysfunction, deficiencies or abnormalities of the mitochondrial oxidative phosphorylation (OXPHOS) system impair cellular energy production. Due to its ability to bypass Complex III and IV of the OXPHOS system and divert electrons from ubiquinol to oxygen, the mitochondrial alternative oxidase (AOX) has drawn attention as a potential -

Glycolysis Citric Acid Cycle Oxidative Phosphorylation Calvin Cycle Light
Stage 3: RuBP regeneration Glycolysis Ribulose 5- Light-Dependent Reaction (Cytosol) phosphate 3 ATP + C6H12O6 + 2 NAD + 2 ADP + 2 Pi 3 ADP + 3 Pi + + 1 GA3P 6 NADP + H Pi NADPH + ADP + Pi ATP 2 C3H4O3 + 2 NADH + 2 H + 2 ATP + 2 H2O 3 CO2 Stage 1: ATP investment ½ glucose + + Glucose 2 H2O 4H + O2 2H Ferredoxin ATP Glyceraldehyde 3- Ribulose 1,5- Light Light Fx iron-sulfur Sakai-Kawada, F Hexokinase phosphate bisphosphate - 4e + center 2016 ADP Calvin Cycle 2H Stroma Mn-Ca cluster + 6 NADP + Light-Independent Reaction Phylloquinone Glucose 6-phosphate + 6 H + 6 Pi Thylakoid Tyr (Stroma) z Fe-S Cyt f Stage 1: carbon membrane Phosphoglucose 6 NADPH P680 P680* PQH fixation 2 Plastocyanin P700 P700* D-(+)-Glucose isomerase Cyt b6 1,3- Pheophytin PQA PQB Fructose 6-phosphate Bisphosphoglycerate ATP Lumen Phosphofructokinase-1 3-Phosphoglycerate ADP Photosystem II P680 2H+ Photosystem I P700 Stage 2: 3-PGA Photosynthesis Fructose 1,6-bisphosphate reduction 2H+ 6 ADP 6 ATP 6 CO2 + 6 H2O C6H12O6 + 6 O2 H+ + 6 Pi Cytochrome b6f Aldolase Plastoquinol-plastocyanin ATP synthase NADH reductase Triose phosphate + + + CO2 + H NAD + CoA-SH isomerase α-Ketoglutarate + Stage 2: 6-carbonTwo 3- NAD+ NADH + H + CO2 Glyceraldehyde 3-phosphate Dihydroxyacetone phosphate carbons Isocitrate α-Ketoglutarate dehydogenase dehydrogenase Glyceraldehyde + Pi + NAD Isocitrate complex 3-phosphate Succinyl CoA Oxidative Phosphorylation dehydrogenase NADH + H+ Electron Transport Chain GDP + Pi 1,3-Bisphosphoglycerate H+ Succinyl CoA GTP + CoA-SH Aconitase synthetase -
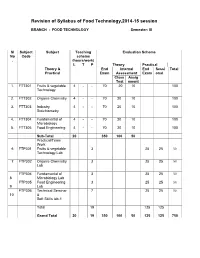
Revision of FT Syllabus Final
Revision of Syllabus of Food Technology,2014-15 session BRANCH : FOOD TECHNOLOGY Semester: III Sl Subject Subject Teaching Evaluation Scheme No Code scheme . (hours/work) L T P Theory Practical Theory & End Internal End Sessi Total Practical Exam Assessment Exam onal Class Assig Test nment 1. FTT301 Fruits & vegetable 4 - - 70 20 10 100 Technology 2. FTT302 Organic Chemistry 4 - - 70 20 10 100 3. FTT303 Industry 4 - - 70 20 10 100 Stoichiometry 4. FTT304 Fundamental of 4 - - 70 20 10 100 Microbiology 5. FTT305 Food Engineering 4 - - 70 20 10 100 Sub-Total 20 350 100 50 Practical/Team Work 6 FTP301 Fruits & vegetable 3 25 25 50 Technology Lab 7 FTP302 Organic Chemistry 3 25 25 50 Lab FTP304 Fundamental of 3 25 25 50 8 Microbiology Lab FTP305 Food Engineering 3 25 25 50 9 Lab FTP306 Technical Seminar 7 25 25 50 10 & Soft Skills lab-1 Total 19 125 125 Grand Total 20 19 350 100 50 125 125 750 FRUITS AND VEGETABLE TECHNOLOGY L T P 4 0 0 III/ FTT-301 Theory Exam. : 3 hrs. Total Contact hrs. : 60 Total Marks : 100 End Exam. : 70 Marks Theory : 60 I.A. : 20 Marks Practical : Nil Assignment : 10 Marks Objective As all the fruits & vegetable are seasonal, their storage, processing, preparation of fruits & vegetable products is highly essential. The students after completion of this paper is well concerned with the storage, preservation, processing & preparation of their products. They also will know details about the preparation of spice powder & condiment products. Topic wise distribution of periods Sl.