Blackbody Radiation (Ch
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Radiant Heating with Infrared
W A T L O W RADIANT HEATING WITH INFRARED A TECHNICAL GUIDE TO UNDERSTANDING AND APPLYING INFRARED HEATERS Bleed Contents Topic Page The Advantages of Radiant Heat . 1 The Theory of Radiant Heat Transfer . 2 Problem Solving . 14 Controlling Radiant Heaters . 25 Tips On Oven Design . 29 Watlow RAYMAX® Heater Specifications . 34 The purpose of this technical guide is to assist customers in their oven design process, not to put Watlow in the position of designing (and guaranteeing) radiant ovens. The final responsibility for an oven design must remain with the equipment builder. This technical guide will provide you with an understanding of infrared radiant heating theory and application principles. It also contains examples and formulas used in determining specifications for a radiant heating application. To further understand electric heating principles, thermal system dynamics, infrared temperature sensing, temperature control and power control, the following information is also available from Watlow: • Watlow Product Catalog • Watlow Application Guide • Watlow Infrared Technical Guide to Understanding and Applying Infrared Temperature Sensors • Infrared Technical Letter #5-Emissivity Table • Radiant Technical Letter #11-Energy Uniformity of a Radiant Panel © Watlow Electric Manufacturing Company, 1997 The Advantages of Radiant Heat Electric radiant heat has many benefits over the alternative heating methods of conduction and convection: • Non-Contact Heating Radiant heaters have the ability to heat a product without physically contacting it. This can be advantageous when the product must be heated while in motion or when physical contact would contaminate or mar the product’s surface finish. • Fast Response Low thermal inertia of an infrared radiation heating system eliminates the need for long pre-heat cycles. -

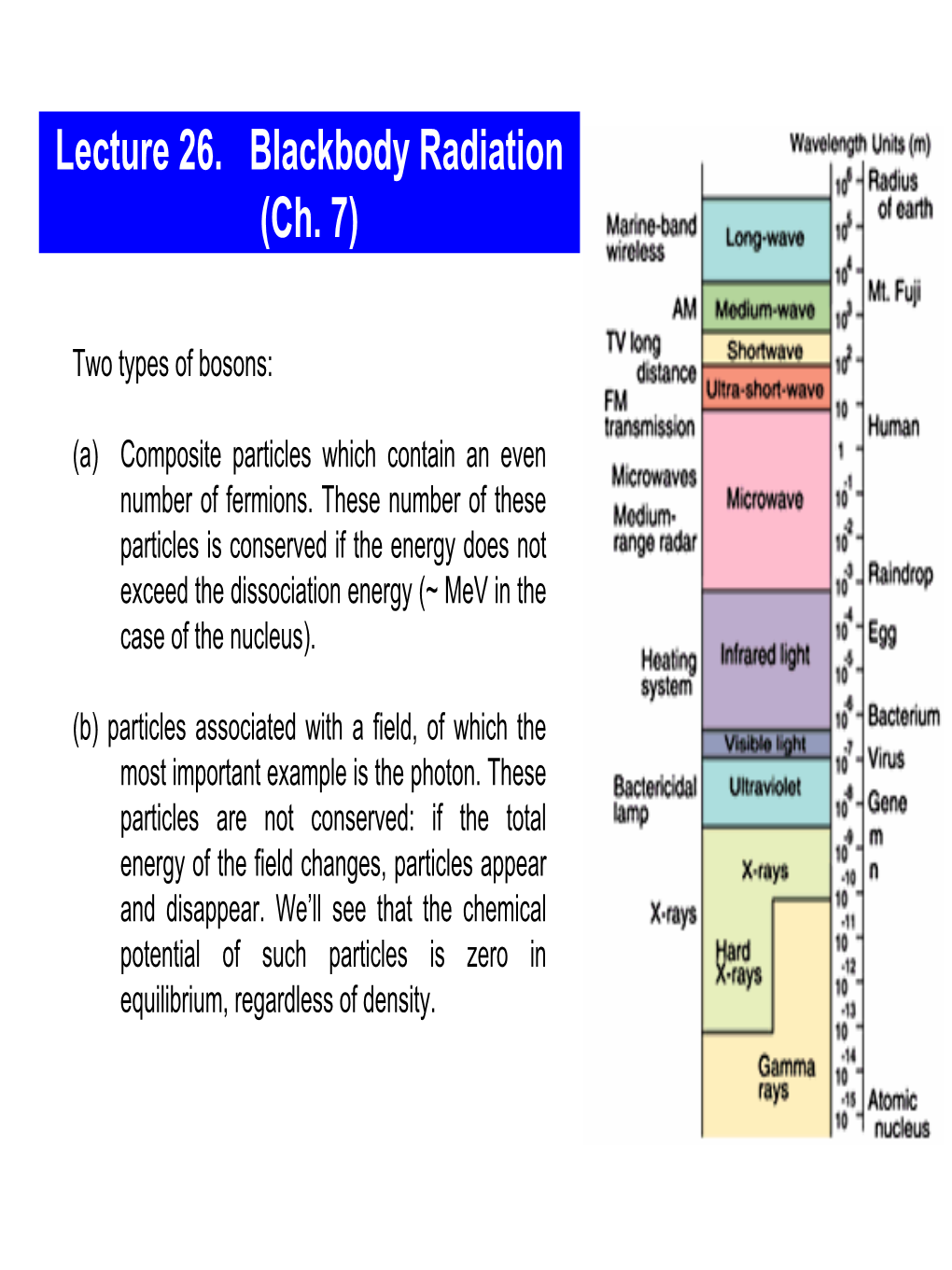
1 Lecture 26
PHYS 445 Lecture 26 - Black-body radiation I 26 - 1 Lecture 26 - Black-body radiation I What's Important: · number density · energy density Text: Reif In our previous discussion of photons, we established that the mean number of photons with energy i is 1 n = (26.1) i eß i - 1 Here, the questions that we want to address about photons are: · what is their number density · what is their energy density · what is their pressure? Number density n Eq. (26.1) is written in the language of discrete states. The first thing we need to do is replace the sum by an integral over photon momentum states: 3 S i ® ò d p Of course, it isn't quite this simple because the density-of-states issue that we introduced before: for every spin state there is one phase space state for every h 3, so the proper replacement more like 3 3 3 S i ® (1/h ) ò d p ò d r The units now work: the left hand side is a number, and so is the right hand side. But this expression ignores spin - it just deals with the states in phase space. For every photon momentum, there are two ways of arranging its polarization (i.e., the orientation of its electric or magnetic field vectors): where the photon momentum vector is perpendicular to the plane. Thus, we have 3 3 3 S i ® (2/h ) ò d p ò d r. (26.2) Assuming that our system is spatially uniform, the position integral can be replaced by the volume ò d 3r = V. -

A Compilation of Data on the Radiant Emissivity of Some Materials at High Temperatures
This is a repository copy of A compilation of data on the radiant emissivity of some materials at high temperatures. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/133266/ Version: Accepted Version Article: Jones, JM orcid.org/0000-0001-8687-9869, Mason, PE and Williams, A (2019) A compilation of data on the radiant emissivity of some materials at high temperatures. Journal of the Energy Institute, 92 (3). pp. 523-534. ISSN 1743-9671 https://doi.org/10.1016/j.joei.2018.04.006 © 2018 Energy Institute. Published by Elsevier Ltd. This is an author produced version of a paper published in Journal of the Energy Institute. Uploaded in accordance with the publisher's self-archiving policy. This manuscript version is made available under the Creative Commons CC-BY-NC-ND 4.0 license http://creativecommons.org/licenses/by-nc-nd/4.0/ Reuse This article is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs (CC BY-NC-ND) licence. This licence only allows you to download this work and share it with others as long as you credit the authors, but you can’t change the article in any way or use it commercially. More information and the full terms of the licence here: https://creativecommons.org/licenses/ Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ A COMPILATION OF DATA ON THE RADIANT EMISSIVITY OF SOME MATERIALS AT HIGH TEMPERATURES J.M Jones, P E Mason and A. -

Bose-Einstein Condensation of Photons and Grand-Canonical Condensate fluctuations
Bose-Einstein condensation of photons and grand-canonical condensate fluctuations Jan Klaers Institute for Applied Physics, University of Bonn, Germany Present address: Institute for Quantum Electronics, ETH Zürich, Switzerland Martin Weitz Institute for Applied Physics, University of Bonn, Germany Abstract We review recent experiments on the Bose-Einstein condensation of photons in a dye-filled optical microresonator. The most well-known example of a photon gas, pho- tons in blackbody radiation, does not show Bose-Einstein condensation. Instead of massively populating the cavity ground mode, photons vanish in the cavity walls when they are cooled down. The situation is different in an ultrashort optical cavity im- printing a low-frequency cutoff on the photon energy spectrum that is well above the thermal energy. The latter allows for a thermalization process in which both tempera- ture and photon number can be tuned independently of each other or, correspondingly, for a non-vanishing photon chemical potential. We here describe experiments demon- strating the fluorescence-induced thermalization and Bose-Einstein condensation of a two-dimensional photon gas in the dye microcavity. Moreover, recent measurements on the photon statistics of the condensate, showing Bose-Einstein condensation in the grandcanonical ensemble limit, will be reviewed. 1 Introduction Quantum statistical effects become relevant when a gas of particles is cooled, or its den- sity is increased, to the point where the associated de Broglie wavepackets spatially over- arXiv:1611.10286v1 [cond-mat.quant-gas] 30 Nov 2016 lap. For particles with integer spin (bosons), the phenomenon of Bose-Einstein condensation (BEC) then leads to macroscopic occupation of a single quantum state at finite tempera- tures [1]. -

Lecture 17 : the Cosmic Microwave Background
Let’s think about the early Universe… Lecture 17 : The Cosmic ! From Hubble’s observations, we know the Universe is Microwave Background expanding ! This can be understood theoretically in terms of solutions of GR equations !Discovery of the Cosmic Microwave ! Earlier in time, all the matter must have been Background (ch 14) squeezed more tightly together ! If crushed together at high enough density, the galaxies, stars, etc could not exist as we see them now -- everything must have been different! !The Hot Big Bang This week: read Chapter 12/14 in textbook 4/15/14 1 4/15/14 3 Let’s think about the early Universe… Let’s think about the early Universe… ! From Hubble’s observations, we know the Universe is ! From Hubble’s observations, we know the Universe is expanding expanding ! This can be understood theoretically in terms of solutions of ! This can be understood theoretically in terms of solutions of GR equations GR equations ! Earlier in time, all the matter must have been squeezed more tightly together ! If crushed together at high enough density, the galaxies, stars, etc could not exist as we see them now -- everything must have been different! ! What was the Universe like long, long ago? ! What were the original contents? ! What were the early conditions like? ! What physical processes occurred under those conditions? ! How did changes over time result in the contents and structure we see today? 4/15/14 2 4/15/14 4 The Poetic Version ! In a brilliant flash about fourteen billion years ago, time and matter were born in a single instant of creation. -

Black Body Radiation and Radiometric Parameters
Black Body Radiation and Radiometric Parameters: All materials absorb and emit radiation to some extent. A blackbody is an idealization of how materials emit and absorb radiation. It can be used as a reference for real source properties. An ideal blackbody absorbs all incident radiation and does not reflect. This is true at all wavelengths and angles of incidence. Thermodynamic principals dictates that the BB must also radiate at all ’s and angles. The basic properties of a BB can be summarized as: 1. Perfect absorber/emitter at all ’s and angles of emission/incidence. Cavity BB 2. The total radiant energy emitted is only a function of the BB temperature. 3. Emits the maximum possible radiant energy from a body at a given temperature. 4. The BB radiation field does not depend on the shape of the cavity. The radiation field must be homogeneous and isotropic. T If the radiation going from a BB of one shape to another (both at the same T) were different it would cause a cooling or heating of one or the other cavity. This would violate the 1st Law of Thermodynamics. T T A B Radiometric Parameters: 1. Solid Angle dA d r 2 where dA is the surface area of a segment of a sphere surrounding a point. r d A r is the distance from the point on the source to the sphere. The solid angle looks like a cone with a spherical cap. z r d r r sind y r sin x An element of area of a sphere 2 dA rsin d d Therefore dd sin d The full solid angle surrounding a point source is: 2 dd sind 00 2cos 0 4 Or integrating to other angles < : 21cos The unit of solid angle is steradian. -

CNT-Based Solar Thermal Coatings: Absorptance Vs. Emittance
coatings Article CNT-Based Solar Thermal Coatings: Absorptance vs. Emittance Yelena Vinetsky, Jyothi Jambu, Daniel Mandler * and Shlomo Magdassi * Institute of Chemistry, The Hebrew University of Jerusalem, Jerusalem 9190401, Israel; [email protected] (Y.V.); [email protected] (J.J.) * Correspondence: [email protected] (D.M.); [email protected] (S.M.) Received: 15 October 2020; Accepted: 13 November 2020; Published: 17 November 2020 Abstract: A novel approach for fabricating selective absorbing coatings based on carbon nanotubes (CNTs) for mid-temperature solar–thermal application is presented. The developed formulations are dispersions of CNTs in water or solvents. Being coated on stainless steel (SS) by spraying, these formulations provide good characteristics of solar absorptance. The effect of CNT concentration and the type of the binder and its ratios to the CNT were investigated. Coatings based on water dispersions give higher adsorption, but solvent-based coatings enable achieving lower emittance. Interestingly, the binder was found to be responsible for the high emittance, yet, it is essential for obtaining good adhesion to the SS substrate. The best performance of the coatings requires adjusting the concentration of the CNTs and their ratio to the binder to obtain the highest absorptance with excellent adhesion; high absorptance is obtained at high CNT concentration, while good adhesion requires a minimum ratio between the binder/CNT; however, increasing the binder concentration increases the emissivity. The best coatings have an absorptance of ca. 90% with an emittance of ca. 0.3 and excellent adhesion to stainless steel. Keywords: carbon nanotubes (CNTs); binder; dispersion; solar thermal coating; absorptance; emittance; adhesion; selectivity 1. -

Measurement of the Cosmic Microwave Background Radiation at 19 Ghz
Measurement of the Cosmic Microwave Background Radiation at 19 GHz 1 Introduction Measurements of the Cosmic Microwave Background (CMB) radiation dominate modern experimental cosmology: there is no greater source of information about the early universe, and no other single discovery has had a greater impact on the theories of the formation of the cosmos. Observation of the CMB confirmed the Big Bang model of the origin of our universe and gave us a look into the distant past, long before the formation of the very first stars and galaxies. In this lab, we seek to recreate this founding pillar of modern physics. The experiment consists of a temperature measurement of the CMB, which is actually “light” left over from the Big Bang. A radiometer is used to measure the intensity of the sky signal at 19 GHz from the roof of the physics building. A specially designed horn antenna allows you to observe microwave noise from isolated patches of sky, without interference from the relatively hot (and high noise) ground. The radiometer amplifies the power from the horn by a factor of a billion. You will calibrate the radiometer to reduce systematic effects: a cryogenically cooled reference load is periodically measured to catch changes in the gain of the amplifier circuit over time. 2 Overview 2.1 History The first observation of the CMB occurred at the Crawford Hill NJ location of Bell Labs in 1965. Arno Penzias and Robert Wilson, intending to do research in radio astronomy at 21 cm wavelength using a special horn antenna designed for satellite communications, noticed a background noise signal in all of their radiometric measurements. -

Black Body Radiation
BLACK BODY RADIATION hemispherical Stefan-Boltzmann law This law states that the energy radiated from a black body is proportional to the fourth power of the absolute temperature. Wien Displacement Law FormulaThe Wien's Displacement Law provides the wavelength where the spectral radiance has maximum value. This law states that the black body radiation curve for different temperatures peaks at a wavelength inversely proportional to the temperature. Maximum wavelength = Wien's displacement constant / Temperature The equation is: λmax= b/T Where: λmax: The peak of the wavelength b: Wien's displacement constant. (2.9*10(−3) m K) T: Absolute Temperature in Kelvin. Emissive power The value of the Stefan-Boltzmann constant is approximately 5.67 x 10 -8 watt per meter squared per kelvin to the fourth (W · m -2 · K -4 ). If the radiation emitted normal to the surface and the energy density of radiation is u, then emissive power of the surface E=c u If the radiation is diffuse Emitted uniformly in all directions 1 E= 푐푢 4 Thermal radiation exerts pressure on the surface on which they are Incident. If the intensity of directed beam of radiations incident normally to The surface is I 퐼 Then Pressure P=u= 푐 If the radiation is diffused 1 P= 푢 3 The value of the constant is approximately 1.366 kilowatts per square metre. When the emissivity of non-black surface is constant at all temperatures and throughout the entire range of wavelength, the surface is called Gray Body. PROBLEMS 1. The temperature of a person’s skin is 350 C. -

Radiation Exchange Between Surfaces
Chapter 1 Radiation Exchange Between Surfaces 1.1 Motivation and Objectives Thermal radiation, as you know, constitutes one of the three basic modes (or mechanisms) of heat transfer, i.e., conduction, convection, and radiation. Actually, on a physical basis, there are only two mechanisms of heat transfer; diffusion (the transfer of heat via molecular interactions) and radiation (the transfer of heat via photons/electomagnetic waves). Convection, being the bulk transport of a fluid, is not precisely a heat transfer mechanism. The physics of radiation transport are distinctly different than diffusion transport. The latter is a local phenomena, meaning that the rate of diffusion heat transfer, at a point in space, precisely depends only on the local nature about the point, i.e., the temperature gradient and thermal conductivity at the point. Of course, the temperature field will depend on the boundary and initial conditions imposed on the system. However, the diffusion heat flux at, say, one point in the system does not directly effect the diffusion flux at some distant point. Radiation, on the other hand, is not local; the flux of radiation at a point will, in general, be directly and instantaneously dependent on the radiation flux at all points in a system. Unlike diffusion, radiation can act over a distance. Accordingly, the mathematical description of radiation transport will employ an integral equation for the radiation field, as opposed to the differential equation for heat diffusion. Our objectives in studying radiation in the short amount of time left in the course will be to 1. Develop a basic physical understanding of electromagnetic radiation, with emphasis on the properties of radiation that are relevant to heat transfer. -

Class 12 I : Blackbody Spectra
Class 12 Spectra and the structure of atoms Blackbody spectra and Wien’s law Emission and absorption lines Structure of atoms and the Bohr model I : Blackbody spectra Definition : The spectrum of an object is the distribution of its observed electromagnetic radiation as a function of wavelength or frequency Particularly important example… A blackbody spectrum is that emitted from an idealized dense object in “thermal equilibrium” You don’t have to memorize this! h=6.626x10-34 J/s -23 kB=1.38x10 J/K c=speed of light T=temperature 1 2 Actual spectrum of the Sun compared to a black body 3 Properties of blackbody radiation… The spectrum peaks at Wien’s law The total power emitted per unit surface area of the emitting body is given by finding the area under the blackbody curve… answer is Stephan-Boltzmann Law σ =5.67X10-8 W/m2/K4 (Stephan-Boltzmann constant) II : Emission and absorption line spectra A blackbody spectrum is an example of a continuum spectrum… it is smooth as a function of wavelength Line spectra: An absorption line is a sharp dip in a (continuum) spectrum An emission line is a sharp spike in a spectrum Both phenomena are caused by the interaction of photons with atoms… each atoms imprints a distinct set of lines in a spectrum The precise pattern of emission/absorption lines tells you about the mix of elements as well as temperature and density 4 Solar spectrum… lots of absorption lines 5 Kirchhoffs laws: A solid, liquid or dense gas produces a continuous (blackbody) spectrum A tenuous gas seen against a hot -

Peter Scicluna – Systematic Effects in Dust-Mass Determinations
The other side of the equation Systematic effects in the determination of dust masses Peter Scicluna ASIAA JCMT Users’ Meeting, Nanjing, 13th February 2017 Peter Scicluna JCMT Users’ Meeting, Nanjing, 13th February 2017 1 / 9 Done for LMC (Reibel+ 2012), SMC (Srinivasan+2016) Total dust mass ∼ integrated dust production over Hubble time What about dust destruction? Dust growth in the ISM? Are the masses really correct? The dust budget crisis: locally Measure dust masses in FIR/sub-mm Measure dust production in MIR Gordon et al., 2014 Peter Scicluna JCMT Users’ Meeting, Nanjing, 13th February 2017 2 / 9 What about dust destruction? Dust growth in the ISM? Are the masses really correct? The dust budget crisis: locally Measure dust masses in FIR/sub-mm Measure dust production in MIR Done for LMC (Reibel+ 2012), SMC (Srinivasan+2016) Total dust mass ∼ integrated dust production over Hubble time Gordon et al., 2014 Peter Scicluna JCMT Users’ Meeting, Nanjing, 13th February 2017 2 / 9 Dust growth in the ISM? Are the masses really correct? The dust budget crisis: locally Measure dust masses in FIR/sub-mm Measure dust production in MIR Done for LMC (Reibel+ 2012), SMC (Srinivasan+2016) Total dust mass ∼ integrated dust production over Hubble time What about dust destruction? Gordon et al., 2014 Peter Scicluna JCMT Users’ Meeting, Nanjing, 13th February 2017 2 / 9 How important are supernovae? Are there even enough metals yet? Dust growth in the ISM? Are the masses really correct? The dust budget crisis: at high redshift Rowlands et al., 2014 Measure