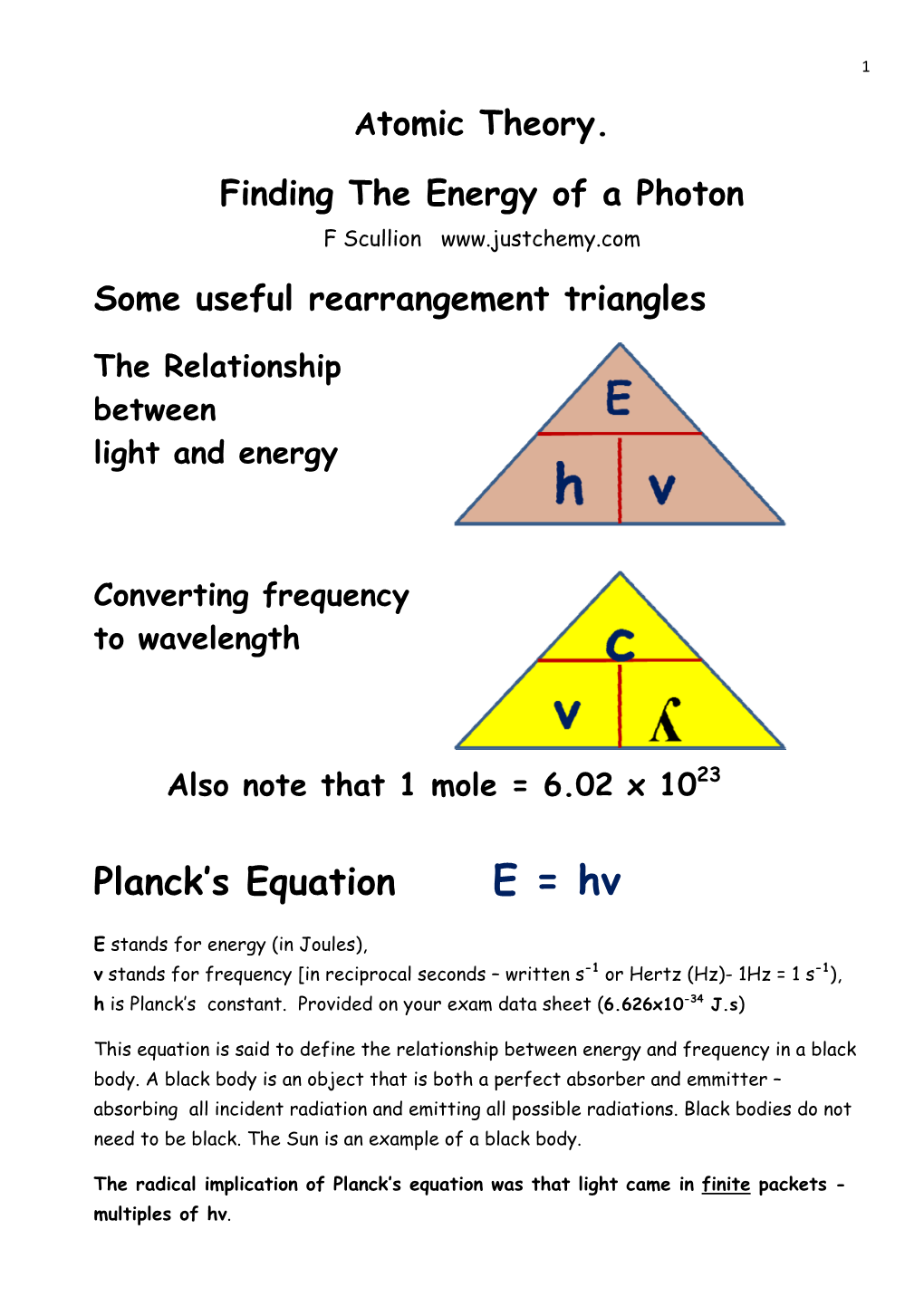
Planck's Equation E = Hv
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary Physics (I-Introduction)
1 Glossary Physics (I-introduction) - Efficiency: The percent of the work put into a machine that is converted into useful work output; = work done / energy used [-]. = eta In machines: The work output of any machine cannot exceed the work input (<=100%); in an ideal machine, where no energy is transformed into heat: work(input) = work(output), =100%. Energy: The property of a system that enables it to do work. Conservation o. E.: Energy cannot be created or destroyed; it may be transformed from one form into another, but the total amount of energy never changes. Equilibrium: The state of an object when not acted upon by a net force or net torque; an object in equilibrium may be at rest or moving at uniform velocity - not accelerating. Mechanical E.: The state of an object or system of objects for which any impressed forces cancels to zero and no acceleration occurs. Dynamic E.: Object is moving without experiencing acceleration. Static E.: Object is at rest.F Force: The influence that can cause an object to be accelerated or retarded; is always in the direction of the net force, hence a vector quantity; the four elementary forces are: Electromagnetic F.: Is an attraction or repulsion G, gravit. const.6.672E-11[Nm2/kg2] between electric charges: d, distance [m] 2 2 2 2 F = 1/(40) (q1q2/d ) [(CC/m )(Nm /C )] = [N] m,M, mass [kg] Gravitational F.: Is a mutual attraction between all masses: q, charge [As] [C] 2 2 2 2 F = GmM/d [Nm /kg kg 1/m ] = [N] 0, dielectric constant Strong F.: (nuclear force) Acts within the nuclei of atoms: 8.854E-12 [C2/Nm2] [F/m] 2 2 2 2 2 F = 1/(40) (e /d ) [(CC/m )(Nm /C )] = [N] , 3.14 [-] Weak F.: Manifests itself in special reactions among elementary e, 1.60210 E-19 [As] [C] particles, such as the reaction that occur in radioactive decay. -

An Atomic Physics Perspective on the New Kilogram Defined by Planck's Constant
An atomic physics perspective on the new kilogram defined by Planck’s constant (Wolfgang Ketterle and Alan O. Jamison, MIT) (Manuscript submitted to Physics Today) On May 20, the kilogram will no longer be defined by the artefact in Paris, but through the definition1 of Planck’s constant h=6.626 070 15*10-34 kg m2/s. This is the result of advances in metrology: The best two measurements of h, the Watt balance and the silicon spheres, have now reached an accuracy similar to the mass drift of the ur-kilogram in Paris over 130 years. At this point, the General Conference on Weights and Measures decided to use the precisely measured numerical value of h as the definition of h, which then defines the unit of the kilogram. But how can we now explain in simple terms what exactly one kilogram is? How do fixed numerical values of h, the speed of light c and the Cs hyperfine frequency νCs define the kilogram? In this article we give a simple conceptual picture of the new kilogram and relate it to the practical realizations of the kilogram. A similar change occurred in 1983 for the definition of the meter when the speed of light was defined to be 299 792 458 m/s. Since the second was the time required for 9 192 631 770 oscillations of hyperfine radiation from a cesium atom, defining the speed of light defined the meter as the distance travelled by light in 1/9192631770 of a second, or equivalently, as 9192631770/299792458 times the wavelength of the cesium hyperfine radiation. -

Non-Einsteinian Interpretations of the Photoelectric Effect
NON-EINSTEINIAN INTERPRETATIONS ------ROGER H. STUEWER------ serveq spectral distribution for black-body radiation, and it led inexorably ~o _the ~latantl~ f::se conclusion that the total radiant energy in a cavity is mfimte. (This ultra-violet catastrophe," as Ehrenfest later termed it, was point~d out independently and virtually simultaneously, though not Non-Einsteinian Interpretations of the as emphatically, by Lord Rayleigh.2) Photoelectric Effect Einstein developed his positive argument in two stages. First, he derived an expression for the entropy of black-body radiation in the Wien's law spectral region (the high-frequency, low-temperature region) and used this expression to evaluate the difference in entropy associated with a chan.ge in volume v - Vo of the radiation, at constant energy. Secondly, he Few, if any, ideas in the history of recent physics were greeted with ~o~s1dere? the case of n particles freely and independently moving around skepticism as universal and prolonged as was Einstein's light quantum ms1d: a given v~lume Vo _and determined the change in entropy of the sys hypothesis.1 The period of transition between rejection and acceptance tem if all n particles accidentally found themselves in a given subvolume spanned almost two decades and would undoubtedly have been longer if v ~ta r~ndomly chosen instant of time. To evaluate this change in entropy, Arthur Compton's experiments had been less striking. Why was Einstein's Emstem used the statistical version of the second law of thermodynamics hypothesis not accepted much earlier? Was there no experimental evi in _c?njunction with his own strongly physical interpretation of the prob dence that suggested, even demanded, Einstein's hypothesis? If so, why ~b1ht~. -

Paul Hertz Astrophysics Subcommittee
Paul Hertz Astrophysics Subcommittee February 23, 2012 Agenda • Science Highlights • Organizational Changes • Program Update • President’s FY13 Budget Request • Addressing Decadal Survey Priorities 2 Chandra Finds Milky Way’s Black Hole Grazing on Asteroids • The giant black hole at the center of the Milky Way may be vaporizing and devouring asteroids, which could explain the frequent flares observed, according to astronomers using data from NASA's Chandra X-ray Observatory. For several years Chandra has detected X-ray flares about once a day from the supermassive black hole known as Sagittarius A*, or "Sgr A*" for short. The flares last a few hours with brightness ranging from a few times to nearly one hundred times that of the black hole's regular output. The flares also have been seen in infrared data from ESO's Very Large Telescope in Chile. • Scientists suggest there is a cloud around Sgr A* containing trillions of asteroids and comets, stripped from their parent stars. Asteroids passing within about 100 million miles of the black hole, roughly the distance between the Earth and the sun, would be torn into pieces by the tidal forces from the black hole. • These fragments then would be vaporized by friction as they pass through the hot, thin gas flowing onto Sgr A*, similar to a meteor heating up and glowing as it falls through Earth's atmosphere. A flare is produced and the remains of the asteroid are eventually swallowed by the black hole. • The authors estimate that it would take asteroids larger than about six miles in radius to generate the flares observed by Chandra. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Electric and Magnetic Fields the Facts
PRODUCED BY ENERGY NETWORKS ASSOCIATION - JANUARY 2012 electric and magnetic fields the facts Electricity plays a central role in the quality of life we now enjoy. In particular, many of the dramatic improvements in health and well-being that we benefit from today could not have happened without a reliable and affordable electricity supply. Electric and magnetic fields (EMFs) are present wherever electricity is used, in the home or from the equipment that makes up the UK electricity system. But could electricity be bad for our health? Do these fields cause cancer or any other disease? These are important and serious questions which have been investigated in depth during the past three decades. Over £300 million has been spent investigating this issue around the world. Research still continues to seek greater clarity; however, the balance of scientific evidence to date suggests that EMFs do not cause disease. This guide, produced by the UK electricity industry, summarises the background to the EMF issue, explains the research undertaken with regard to health and discusses the conclusion reached. Electric and Magnetic Fields Electric and magnetic fields (EMFs) are produced both naturally and as a result of human activity. The earth has both a magnetic field (produced by currents deep inside the molten core of the planet) and an electric field (produced by electrical activity in the atmosphere, such as thunderstorms). Wherever electricity is used there will also be electric and magnetic fields. Electric and magnetic fields This is inherent in the laws of physics - we can modify the fields to some are inherent in the laws of extent, but if we are going to use electricity, then EMFs are inevitable. -

Relationships of the SI Derived Units with Special Names and Symbols and the SI Base Units
Relationships of the SI derived units with special names and symbols and the SI base units Derived units SI BASE UNITS without special SI DERIVED UNITS WITH SPECIAL NAMES AND SYMBOLS names Solid lines indicate multiplication, broken lines indicate division kilogram kg newton (kg·m/s2) pascal (N/m2) gray (J/kg) sievert (J/kg) 3 N Pa Gy Sv MASS m FORCE PRESSURE, ABSORBED DOSE VOLUME STRESS DOSE EQUIVALENT meter m 2 m joule (N·m) watt (J/s) becquerel (1/s) hertz (1/s) LENGTH J W Bq Hz AREA ENERGY, WORK, POWER, ACTIVITY FREQUENCY second QUANTITY OF HEAT HEAT FLOW RATE (OF A RADIONUCLIDE) s m/s TIME VELOCITY katal (mol/s) weber (V·s) henry (Wb/A) tesla (Wb/m2) kat Wb H T 2 mole m/s CATALYTIC MAGNETIC INDUCTANCE MAGNETIC mol ACTIVITY FLUX FLUX DENSITY ACCELERATION AMOUNT OF SUBSTANCE coulomb (A·s) volt (W/A) C V ampere A ELECTRIC POTENTIAL, CHARGE ELECTROMOTIVE ELECTRIC CURRENT FORCE degree (K) farad (C/V) ohm (V/A) siemens (1/W) kelvin Celsius °C F W S K CELSIUS CAPACITANCE RESISTANCE CONDUCTANCE THERMODYNAMIC TEMPERATURE TEMPERATURE t/°C = T /K – 273.15 candela 2 steradian radian cd lux (lm/m ) lumen (cd·sr) 2 2 (m/m = 1) lx lm sr (m /m = 1) rad LUMINOUS INTENSITY ILLUMINANCE LUMINOUS SOLID ANGLE PLANE ANGLE FLUX The diagram above shows graphically how the 22 SI derived units with special names and symbols are related to the seven SI base units. In the first column, the symbols of the SI base units are shown in rectangles, with the name of the unit shown toward the upper left of the rectangle and the name of the associated base quantity shown in italic type below the rectangle. -

1. Physical Constants 1 1
1. Physical constants 1 1. PHYSICAL CONSTANTS Table 1.1. Reviewed 1998 by B.N. Taylor (NIST). Based mainly on the “1986 Adjustment of the Fundamental Physical Constants” by E.R. Cohen and B.N. Taylor, Rev. Mod. Phys. 59, 1121 (1987). The last group of constants (beginning with the Fermi coupling constant) comes from the Particle Data Group. The figures in parentheses after the values give the 1-standard- deviation uncertainties in the last digits; the corresponding uncertainties in parts per million (ppm) are given in the last column. This set of constants (aside from the last group) is recommended for international use by CODATA (the Committee on Data for Science and Technology). Since the 1986 adjustment, new experiments have yielded improved values for a number of constants, including the Rydberg constant R∞, the Planck constant h, the fine- structure constant α, and the molar gas constant R,and hence also for constants directly derived from these, such as the Boltzmann constant k and Stefan-Boltzmann constant σ. The new results and their impact on the 1986 recommended values are discussed extensively in “Recommended Values of the Fundamental Physical Constants: A Status Report,” B.N. Taylor and E.R. Cohen, J. Res. Natl. Inst. Stand. Technol. 95, 497 (1990); see also E.R. Cohen and B.N. Taylor, “The Fundamental Physical Constants,” Phys. Today, August 1997 Part 2, BG7. In general, the new results give uncertainties for the affected constants that are 5 to 7 times smaller than the 1986 uncertainties, but the changes in the values themselves are smaller than twice the 1986 uncertainties. -

Evaluating Temperature Regulation by Field-Active Ectotherms: the Fallacy of the Inappropriate Question
Vol. 142, No. 5 The American Naturalist November 1993 EVALUATING TEMPERATURE REGULATION BY FIELD-ACTIVE ECTOTHERMS: THE FALLACY OF THE INAPPROPRIATE QUESTION *Department of Biological Sciences, Barnard College, Columbia University, New York, New York 10027; $Department of Zoology NJ-15, University of Washington, Seattle, Washington 98195; $Department of Biology, University of Massachusetts at Boston, Boston, Massachusetts 02125 Submitted March 16, 1992; Revised November 9, 1992; Accepted November 20, 1992 Abstract.-We describe a research protocol for evaluating temperature regulation from data on small field-active ectothermic animals, especially lizards. The protocol requires data on body temperatures (T,) of field-active ectotherms, on available operative temperatures (T,, "null temperatures" for nonregulating animals), and on the thermoregulatory set-point range (pre- ferred body temperatures, T,,,). These data are used to estimate several quantitative indexes that collectively summarize temperature regulation: the "precision" of body temperature (vari- ance in T,, or an equivalent metric), the "accuracy" of body temperature relative to the set-point range (the average difference between 7, and T,,,), and the "effectiveness" of thermoregulation (the extent to which body temperatures are closer on the average to the set-point range than are operative temperatures). If additional data on the thermal dependence of performance are available, the impact of thermoregulation on performance (the extent to which performance is enhanced relative to that of nonregulating animals) can also be estimated. A sample analysis of the thermal biology of three Anolis lizards in Puerto Rico demonstrates the utility of the new protocol and its superiority to previous methods of evaluating temperature regulation. We also discuss several ways in which the research protocol can be extended and applied to other organisms. -

Determination of Planck's Constant Using the Photoelectric Effect
Determination of Planck’s Constant Using the Photoelectric Effect Lulu Liu (Partner: Pablo Solis)∗ MIT Undergraduate (Dated: September 25, 2007) Together with my partner, Pablo Solis, we demonstrate the particle-like nature of light as charac- terized by photons with quantized and distinct energies each dependent only on its frequency (ν) and scaled by Planck’s constant (h). Using data obtained through the bombardment of an alkali metal surface (in our case, potassium) by light of varying frequencies, we calculate Planck’s constant, h to be 1.92 × 10−15 ± 1.08 × 10−15eV · s. 1. THEORY AND MOTIVATION This maximum kinetic energy can be determined by applying a retarding potential (Vr) across a vacuum gap It was discovered by Heinrich Hertz that light incident in a circuit with an amp meter. On one side of this gap upon a matter target caused the emission of electrons is the photoelectron emitter, a metal with work function from the target. The effect was termed the Hertz Effect W0. We let light of different frequencies strike this emit- (and later the Photoelectric Effect) and the electrons re- ter. When eVr = Kmax we will cease to see any current ferred to as photoelectrons. It was understood that the through the circuit. By finding this voltage we calculate electrons were able to absorb the energy of the incident the maximum kinetic energy of the electrons emitted as a light and escape from the coulomb potential that bound function of radiation frequency. This relationship (with it to the nucleus. some variation due to error) should model Equation 2 According to classical wave theory, the energy of a above. -

Quantifying the Quantum Stephan Schlamminger Looks at the Origins of the Planck Constant and Its Current Role in Redefining the Kilogram
measure for measure Quantifying the quantum Stephan Schlamminger looks at the origins of the Planck constant and its current role in redefining the kilogram. child on a swing is an everyday mechanical constant (h) with high precision, measure a approximation of a macroscopic a macroscopic experiment is necessary, force — in A harmonic oscillator. The total energy because the unit of mass, the kilogram, can this case of such a system depends on its amplitude, only be accessed with high precision at the the weight while the frequency of oscillation is, at one-kilogram value through its definition of a mass least for small amplitudes, independent as the mass of the International Prototype m — as of the amplitude. So, it seems that the of the Kilogram (IPK). The IPK is the only the product energy of the system can be adjusted object on Earth whose mass we know for of a current continuously from zero upwards — that is, certain2 — all relative uncertainties increase and a voltage the child can swing at any amplitude. This from thereon. divided by a velocity, is not the case, however, for a microscopic The revised SI, likely to come into mg = UI/v (with g the oscillator, the physics of which is described effect in 2019, is based on fixed numerical Earth’s gravitational by the laws of quantum mechanics. A values, without uncertainties, of seven acceleration). quantum-mechanical swing can change defining constants, three of which are Meanwhile, the its energy only in steps of hν, the product already fixed in the present SI; the four discoveries of the Josephson of the Planck constant and the oscillation additional ones are the elementary and quantum Hall effects frequency — the (energy) quantum of charge and the Planck, led to precise electrical the oscillator. -

The International System of Units (SI)
NAT'L INST. OF STAND & TECH NIST National Institute of Standards and Technology Technology Administration, U.S. Department of Commerce NIST Special Publication 330 2001 Edition The International System of Units (SI) 4. Barry N. Taylor, Editor r A o o L57 330 2oOI rhe National Institute of Standards and Technology was established in 1988 by Congress to "assist industry in the development of technology . needed to improve product quality, to modernize manufacturing processes, to ensure product reliability . and to facilitate rapid commercialization ... of products based on new scientific discoveries." NIST, originally founded as the National Bureau of Standards in 1901, works to strengthen U.S. industry's competitiveness; advance science and engineering; and improve public health, safety, and the environment. One of the agency's basic functions is to develop, maintain, and retain custody of the national standards of measurement, and provide the means and methods for comparing standards used in science, engineering, manufacturing, commerce, industry, and education with the standards adopted or recognized by the Federal Government. As an agency of the U.S. Commerce Department's Technology Administration, NIST conducts basic and applied research in the physical sciences and engineering, and develops measurement techniques, test methods, standards, and related services. The Institute does generic and precompetitive work on new and advanced technologies. NIST's research facilities are located at Gaithersburg, MD 20899, and at Boulder, CO 80303.