Apbrprelim 2..10
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

THE SOCIAL ORGANIZATION and MATING SYSTEM of the STRIATED GRASSWREN Author(S): Jordan Karubian Source: the Condor, 103(2):412-417
THE SOCIAL ORGANIZATION AND MATING SYSTEM OF THE STRIATED GRASSWREN Author(s): Jordan Karubian Source: The Condor, 103(2):412-417. 2001. Published By: Cooper Ornithological Society DOI: http://dx.doi.org/10.1650/0010-5422(2001)103[0412:TSOAMS]2.0.CO;2 URL: http://www.bioone.org/doi/full/10.1650/0010-5422%282001%29103%5B0412%3ATSOAMS %5D2.0.CO%3B2 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. SHORT COMMUNICATIONS 405 The Condor 103:405±408 q The Cooper Ornithological Society 2001 SPECIES AND SEX-BIASED PREDATION ON HATCHLING GREEN TURTLES BY FRIGATEBIRDS ON EUROPA ISLAND, WESTERN INDIAN OCEAN1 FRE DE RIC LAGARDE2 DeÂpartement de Biologie, Universite des Sciences et Techniques, Avenue Marcillac, 17000 La Rochelle, France MATTHIEU LE CORRE Laboratoire d'Ecologie Marine, UniversiteÂdeLaReÂunion, 97715 Saint Denis cedex, ReÂunion Island, France HERVE LORMEE Centre d'eÂtudes biologiques de ChizeÂ, C.N.R.S, 79360 Villiers en Bois, France Abstract. -

Amytornis Modestus Inexpectuatus Thick Billed Grasswren
NSW Threatened Species Scientific Committee Conservation Assessment of Thick billed Grasswren Amytornis modestus inexpectatus (Matthews, 1912) (Maluridae) B Hope, A Kerle, April 2020 NSW Threatened Species Scientific Committee Thick-billed Grasswren Amytornis modestus inexpectatus (Matthews, 1912) Distribution: endemic to NSW; [Amytornis modestus occurs in the Northern Territory, South Australia and NSW; 1 other subspecies in NSW]. Current EPBC Act Status: Amytornis modestus listed as Vulnerable (subspecies not listed) Current NSW BC Act Status: Amytornis modestus inexpectatus listed as Critically Endangered Proposed listing on NSW BC Act and EPBC Act: Extinct Conservation Advice: Thick-billed Grasswren Amytornis modestus inexpectatus (Matthews, 1912) Summary of Conservation Assessment Amytornis modestus inexpectatus (Matthews, 1912) (Thick-billed Grasswren) found to be eligible for listing as extinct as at the time of this review there is no reasonable doubt that the last member of the species has died. Exhaustive surveys in known and/or expected habitat, at appropriate times (diurnal, seasonal, annual), and throughout its historical range have failed to record any individuals. Habitat change within the inferred range of this species has been substantial and remaining suitable habitat is uncommon and considered to be comprehensively surveyed. Description and Taxonomy Amytornis modestus (North, 1902) (Thick-billed Grasswren) is one of 11 presently recognised species within the purely continental Australian genus Amytornis (Black 2016). Until recently the species A. textilis was recognised across Australia, however since 2010 the eastern and western populations have been placed in A.m modestus (Thick-billed Grasswren) and Amytornis textilis (Western Grasswren), respectively (Christidis et al. 2010). A recent taxonomic assessment identified seven subspecies of A. -

Australia South Australian Outback 8Th June to 23Rd June 2021 (13 Days)
Australia South Australian Outback 8th June to 23rd June 2021 (13 days) Splendid Fairywren by Dennis Braddy RBL South Australian Outback Itinerary 2 Nowhere is Australia’s vast Outback country more varied, prolific and accessible than in the south of the country. Beginning and ending in Adelaide, we’ll traverse the region’s superb network of national parks and reserves before venturing along the remote, endemic-rich and legendary Strzelecki and Birdsville Tracks in search of a wealth of Australia’s most spectacular, specialised and enigmatic endemics such as Grey and Black Falcons, Letter-winged Kite, Black-breasted Buzzard, Chestnut- breasted and Banded Whiteface, Gibberbird, Yellow, Crimson and Orange Chats, Inland Dotterel, Flock Bronzewing, spectacular Scarlet-chested and Regent Parrots, Copperback and Cinnamon Quail- thrushes, Banded Stilt, White-browed Treecreeper, Red-lored and Gilbert’s Whistlers, an incredible array of range-restricted Grasswrens, the rare and nomadic Black and Pied Honeyeaters, Black-eared Cuckoo and the incredible Major Mitchell’s Cockatoo. THE TOUR AT A GLANCE… THE SOUTH AUTRALIAN OUTBACK ITINERARY Day 1 Arrival in Adelaide Day 2 Adelaide to Berri Days 3 & 4 Glue Pot Reserve and Calperum Station Day 5 Berri to Wilpena Pound and Flinders Ranges National Park Day 6 Wilpena Pound to Lyndhurst Day 7 Strzelecki Track Day 8 Lyndhurst to Mungerranie via Marree and Birdsville Track Day 9 Mungerranie and Birdsville Track area Day 10 Mungerranie to Port Augusta Day 11 Port Augusta area Day 12 Port Augusta to Adelaide Day 13 Adelaide and depart RBL South Australian Outback Itinerary 3 TOUR MAP… RBL South Australian Outback Itinerary 4 THE TOUR IN DETAIL… Day 1. -

REMARKS on the TYMPANIC CAVITY of MALURUS, STIPITURUS and AMYTORNIS (PASSERIFORMES, MALURIDAE) S
SEPTEMBER, 1982 17 REMARKS ON THE TYMPANIC CAVITY OF MALURUS, STIPITURUS AND AMYTORNIS (PASSERIFORMES, MALURIDAE) s. A. PARKER INTRODUCTION THE AVIAN TYMPANIC CAVITY Mayr & Amadon (1951) and Keast (1961) In mammals, the tympanic cavity or middle recognized the subfamily Malurinae for a group ear is usually more or less entirely enclosed by of Australasian wren- and warbler-like genera, bone to form the auditory bulla (see for instance including Malurus, Stipiturus, Todopsis, Cheno Novacek 1977). In birds, however, it is usually rhamphus, Clytomyias, Dasyornis, Amytornis, merely a shallow concavity in the skull, bounded Aphelocephala, Sericornis, Acanthiza and Gery posteriorly by the ala tympanica (tympanic gone. Harrison & Parker (1965), chiefly on wing), a lateral flaring of the os exoccipitale, behavioural evidence, restricted the subfamily and ventrally by a much smaller extension of to include only the first five genera and the the os parasphenoidale also termed the ala Fijian genus Lamprolia, and used the term tympanica (Baumel 1979: 82, 88). In the skull Acanthizinae to cover the remainder. Subse of the Common or American Crow Corvus quently, Harrison (1969) redefined the Malur brachyrhynchos (Baumel 1979: 109) and in the inae sensu stricto, including Amytornis and ex skulls of all five Australian species of Corvus cluding Lamprolia (which latter may actually (including the Little Crow C. bennetti, fig. 4b), be a monarchine flycatcher fide Olson 1980). the exoccipital tympanic wing is not well Schodde (1975) raised the Malurinae of developed, providing little more than a posterior Harrison to the rank of family, the Maluridae, wall to a' quite open tympanic cavity. In the a move that emphasizes the uncertainty concern skulls of other Australian passerines examined ing the group's taxonomic relationships. -

The Behavioural Ecology of the Thick-Billed Grasswren
The behavioural ecology of the thick-billed grasswren Marina (Maria Carolina Johanna) Louter (MSc Biology) A thesis submitted in fulfilment of the requirements for the Degree of Doctor of Philosophy School of Biological Sciences Faculty of Science and Engineering Flinders University of South Australia Cover image: Typical thick-billed grasswren habitat with chenopod shrubs at Witchelina Nature Reserve in South Australia, and (inset) a thick-billed grasswren (Amytornis modestus raglessi) in the hand. Photos by Marina Louter. ii Table of Contents List of Tables ................................................................................................................... vii List of Figures ................................................................................................................... ix List of Supplementary Material ..................................................................................... xi Thesis Summary .............................................................................................................. xii Declaration...................................................................................................................... xiv Acknowledgements ......................................................................................................... xv Statement of Authorship/Contribution and Acknowledgment ............................... xviii Chapter 1 General introduction ................................................................ 1 Behavioural conservation framework ................................................................... -

Structure and Function of the Cloacal Tip of Male Australian Maluridae
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln U.S. Department of Agriculture: Agricultural Publications from USDA-ARS / UNL Faculty Research Service, Lincoln, Nebraska 2008 Good vibrations? Structure and function of the cloacal tip of male Australian Maluridae Melissah Rowe University of Chicago, [email protected] Murray R. Bakst USDA Stephen Pruett-Jones University of Chicago Follow this and additional works at: https://digitalcommons.unl.edu/usdaarsfacpub Part of the Agricultural Science Commons Rowe, Melissah; Bakst, Murray R.; and Pruett-Jones, Stephen, "Good vibrations? Structure and function of the cloacal tip of male Australian Maluridae" (2008). Publications from USDA-ARS / UNL Faculty. 626. https://digitalcommons.unl.edu/usdaarsfacpub/626 This Article is brought to you for free and open access by the U.S. Department of Agriculture: Agricultural Research Service, Lincoln, Nebraska at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Publications from USDA-ARS / UNL Faculty by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. J. Avian Biol. 39: 348Á354, 2008 doi: 10.1111/j.2008.0908-8857.04305.x # 2008 The Authors. J. Compilation # 2008 J. Avian Biol. Received 2 July 2007, accepted 1 October 2007 Good vibrations? Structure and function of the cloacal tip of male Australian Maluridae Melissah Rowe, Murray R. Bakst and Stephen Pruett-Jones M. Rowe (correspondence) and S. Pruett-Jones, Department of Ecology and Evolution, University of Chicago, 1101 East 57th Street, Chicago, Illinois 60637, USA. Email: [email protected]. Á M. R. Bakst, Animal Biosciences and Biotechnology Laboratory, Animal and Natural Resources Institute, Agricultural Research Service, USDA, Building 200, BARC East, Powder Mill Road, Beltsville, Maryland 20705, USA. -

Observations of the Striated Grasswren Amytornis Striatus Rowleyi at Opalton, Central Western Queensland
Australian Field Ornithology 2014, 31, 17–23 Observations of the Striated Grasswren Amytornis striatus rowleyi at Opalton, central western Queensland K.A. Wood 8 Kalamunda Street, North Lakes QLD 4509, Australia Email: [email protected] Summary. Ninety-six records of the Striated Grasswren Amytornis striatus rowleyi (Rusty Grasswren A. rowleyi) were obtained during 14 visits to Opalton in central western Queensland between May 2009 and October 2012. Most records (35) were of groups with a minimum of two individuals (range 1–9+). Three calls are described: contact call, song and alarm. They were uttered throughout the day from first light to sunset. In 12 records, Striated Grasswrens were seen to fly, usually 30–40 cm above the ground over a median distance of 15 m (range 8–35 m). Groups of Striated Grasswrens were associated with Rufous-crowned Emu-wrens Stipiturus ruficeps in 15 records. Inquisitive behaviour is described, and other behaviours Forum— are compared with co-occurring Rufous-crowned Emu-wrens (34 groups) and Spinifexbirds Eremiornis carteri (19 individuals). Do Tasmanian Southern Boobooks migrate? Introduction The Amytornis grasswrens are among Australia’s most elusive and least known Jerry Olsen1* and S.J.S. Debus2 birds (Rowley & Russell 1997; Christidis et al. 2010). They are cryptic, shy and secretive (Pringle 1982; Chapman 1996; Karubian 2001) and mostly live in 1Institute for Applied Ecology, University of Canberra, Canberra, ACT 2601, Australia remote parts of Australia. The north-eastern subspecies of the Striated Grasswren 2Zoology, University of New England, Armidale, NSW 2351, Australia A. striatus rowleyi is no exception. It was first collected near Opalton in 1970 Corresponding author. -

Amytornis Textilis )
Amytornis 1 WESTER A USTRALIA J OURAL OF O RITHOLOGY Volume 3 (2011) 1-12 ARTICLE Western Australia, home of the Grass-Wren ( Amytornis textilis ) Andrew Black Ornithology Section, South Australian Museum, North Terrace, Adelaide, South Australia, Australia 5000. Email: [email protected] Abstract. The first grasswrens to be seen by Europeans, at Shark Bay, were given the English name Textile Wren, later the Grass-Wren. Though detected subsequently in many other places in southern Western Australia they then declined dramatically and soon disappeared from all but the place of their original discovery. Specimens collected many hundreds of kilometres apart and in varying environments showed differences that led to their be- ing given many separate names. They were shortly dispersed among Australian and later among North American institutions with none having a fully representative collection. Subsequent extinctions restricted the opportunity to confirm or modify this implicit taxonomic diversity. From evidence presented here I propose that two Western Australian subspecies be recognised as separate, Amytornis textilis textilis of the Shark Bay region and arid north- ern interior and A. t. macrourus of southern eucalypt communities. Keywords . Western Grasswren, morphological diversity, habitat diversity, taxonomy, subspecies macrourus rec- ognised. Introduction lution of the Australian arid zone fauna. In an earlier analysis of the confounding taxonomy The first grasswrens, genus Amytornis (Maluridae), to of the grasswrens Amytornis textilis , modestus and be given scientific description were obtained in 1818 at purnelli Parker (1972) wrote: "The Western Australian Shark Bay Western Australia by Quoy and Gaimard records of textilis will be discussed by Mr. J. Ford (in (1824). -

Australia's Biodiversity – Responses to Fire
AUSTRALIA’S BIODIVERSITY – RESPONSES TO FIRE Plants, birds and invertebrates A.M. Gill, J.C.Z. Woinarski, A. York Biodiversity Technical Paper, No. 1 Cover photograph credits Group of 3 small photos, front cover: • Cockatiel. The Cockatiel is one of a group of highly mobile birds which track resource-rich areas. These areas fluctuate across broad landscapes in response to local rainfall or fire events. Large flocks may congregate on recently-burnt areas. /Michael Seyfort © Nature Focus • Fern regeneration post-fire, Clyde Mountain, NSW, 1988. /A. Malcolm Gill • These bull ants (Myrmecia gulosa) are large ants which generally build small mounds and prefer open areas in which to forage for food. They are found on frequently burnt sites. Despite their fierce appearance, they feed mainly on plant products. /Alan York. Small photo, lower right, front cover: • Fuel reduction burning in dry forest. This burn is towards the “hotter” end of the desirable range. /Alan York Large photo on spine: • Forest fire, Kapalga, NT, 1990. /Malcolm Gill Small photo, back cover: • Cycad response after fire near Darwin, NT. /Malcolm Gill ISBN 0 642 21422 0 Published by the Department of the Environment and Heritage © Commonwealth of Australia, 1999 Information presented in this document may be copied for personal use or pub- lished for educational purposes, provided that any extracts are acknowledged. The views expressed in this paper are those of the authors and do not necessarily represent the views of the Department, or of the Commonwealth of Australia. Biodiversity Convention and Strategy Section Department of the Environment and Heritage GPO Box 636 CANBERRA ACT 2601 General enquiries, telephone 1800 803772 Design: Design One Solutions, Canberra Printing: Goanna Print, Canberra Printed in Australia on recycled Australian paper AUSTRALIA’S BIODIVERSITY – RESPONSES TO FIRE Plants, birds and invertebrates A. -
SBOC Full Bird List
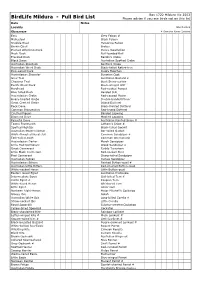
Box 1722 Mildura Vic 3502 BirdLife Mildura - Full Bird List Please advise if you see birds not on this list Date Notes Locality March 2012 Observers # Denotes Rarer Species Emu Grey Falcon # Malleefowl Black Falcon Stubble Quail Peregrine Falcon Brown Quail Brolga Plumed Whistling-Duck Purple Swamphen Musk Duck Buff-banded Rail Freckled Duck Baillon's Crake Black Swan Australian Spotted Crake Australian Shelduck Spotless Crake Australian Wood Duck Black-tailed Native-hen Pink-eared Duck Dusky Moorhen Australasian Shoveler Eurasian Coot Grey Teal Australian Bustard # Chestnut Teal Bush Stone-curlew Pacific Black Duck Black-winged Stilt Hardhead Red-necked Avocet Blue-billed Duck Banded Stilt Australasian Grebe Red-capped Plover Hoary-headed Grebe Double-banded Plover Great Crested Grebe Inland Dotterel Rock Dove Black-fronted Dotterel Common Bronzewing Red-kneed Dotterel Crested Pigeon Banded Lapwing Diamond Dove Masked Lapwing Peaceful Dove Australian Painted Snipe # Tawny Frogmouth Latham's Snipe # Spotted Nightjar Black-tailed Godwit Australian Owlet-nightjar Bar-tailed Godwit White-throated Needletail Common Sandpiper # Fork-tailed Swift Common Greenshank Australasian Darter Marsh Sandpiper Little Pied Cormorant Wood Sandpiper # Great Cormorant Ruddy Turnstone Little Black Cormorant Red-necked Stint Pied Cormorant Sharp-tailed Sandpiper Australian Pelican Curlew Sandpiper Australasian Bittern Painted Button-quail # Australian Little Bittern Red-chested Button-quail White-necked Heron Little Button-quail Eastern Great Egret Australian -

Supplementary Methods S1
1 Validation methods for trophic niche models 2 3 To assign links between nodes (species), we used trophic niche-space models (e.g., [1]). 4 Each of these models has two quantile regressions that define the prey-size range a 5 predator of a given size is predicted to consume. Species whose body mass is within the 6 range of a predator’s prey size, as identified by the trophic niche-space model, are predicted 7 to be prey, while those outside the range are predicted not to be eaten. 8 9 The broad taxonomy of a predator helps to predict predation interactions [2]. To optimize 10 our trophic niche-space model, we therefore tested whether including taxonomic class of 11 predators improved the fit of quantile regressions. Using trophic (to identify which species 12 were predators), body mass, and taxonomic data, we fitted and compared five quantile 13 regression models (including a null model) to the GloBI data. In each model, we log10- 14 transformed the dependent variable prey body mass, and included for the independent 15 variables different combinations of log10-transformed predator body mass, predator class, 16 and the interaction between these variables (Supplementary Table S4). We log10- 17 transformed both predator and prey body mass to linearize the relationship between these 18 variables. We fit the five quantile regressions to the upper and lower 5% of prey body mass, 19 and compared model fits using the Bayesian information criterion (BIC). The predator body 20 mass*predator class model fit the 95th quantile data best, whereas the predator body mass 21 + predator class model fit the 5th quantile data marginally better than the aforementioned 22 interaction model (Supplementary Figure S2, Supplementary Table S4). -

Recent Observations of the Thick-Billed Grasswren Amytornis Textilis Modestus in New South Wales
159 AUSTRALIAN FIELD ORNITHOLOGY 2010, 27, 159–164 Recent Observations of the Thick-billed Grasswren Amytornis textilis modestus in New South Wales DAVID G. PARKER1, DAVID EGAN2 AND MICHELLE L. BALLESTRIN2 1Department of Environment, Climate Change and Water, P.O. Box 397, Griffith, New South Wales 2680 (Email: [email protected]) 2Department of Environment, Climate Change and Water, Parks and Wildlife Group, P.O. Box 1049, Griffith, New South Wales 2680 Summary This note provides details of two reports of the Thick-billed Grasswren Amytornis textilis modestus in north-western New South Wales in August 2008 and August 2009. These observations were of birds on a low stony ridgeline with sparse Black Bluebush Maireana pyramidata and Thorny Saltbush Rhagodia spinescens shrubland ~80 km east of the nearest historical record at Mount Arrowsmith Station, NSW. Introduction The Thick-billed Grasswren Amytornis textilis inhabits shrublands of the arid zone of Australia. Three subspecies are recognised: A.t. textilis from Western Australia (WA), where it is now restricted to the Shark Bay area; A.t. myall from South Australia (SA), between Whyalla and the Gawler Ranges (Higgins et al. 2001; Johnstone & Storr 2004; Black et al. 2009); and the eastern subspecies A.t. modestus, which formerly extended from the southern Northern Territory (NT) through northern-central SA into north-western New South Wales (NSW) (McAllan 1987, 2000; Higgins et al. 2001; NPWS 2002). The species has, however, suffered a substantial reduction in distribution since European settlement, attributed in parts of its range to the arrival of Rabbits Oryctolagus cuniculus (Johnstone & Storr 2004).