Toxicological Profile for Atrazine
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Specific Target Organ Systemic Toxicity (Tost) Following Repeated Exposure
Chapter 13: SPECIFIC TARGET ORGAN SYSTEMIC TOXICITY (TOST) FOLLOWING REPEATED EXPOSURE DEFINITIONS 1. Classification identifies the chemical substance as being a specific target organ/systemic toxicant and, as such, it may present a potential for adverse health impact to people who are exposed to it. 2. Classification depends upon the availability of reliable evidence that repeated exposure to the substance has produced a consistent and identifiable toxic effect in humans, or, in experimental animals, toxicologically significant changes which have affected the function or morphology of a tissue/organ, or has produced serious changes to the biochemistry or haematology of the organism and these changes are relevant for human health. 3. Assessment of specific target organ/systemic toxicity should take into consideration not only significant changes in a single organ or biological system but also generalised changes of a less severe nature involving several organs. CONSIDERATIONS 4. The purpose of this document is to provide a means of classifying substances that produce specific target organ/systemic toxicity arising from repeated exposure that is not specifically addressed elsewhere in the harmonised classification system (GHS). All significant health effects that can impair function, both reversible and irreversible, following repeated or long-term exposure, are included. Other specific toxic effects, such as acute lethality/toxicity, eye and skin corrosivity/irritation, skin and respiratory sensitisation, carcinogenicity, mutagenicity and reproductive toxicity are assessed separately in the GHS and consequently are not included in the present proposal. 5. Non-lethal toxic effects observed after a single-event exposure are classified elsewhere in the GHS as a separate chapter and, therefore, are excluded from the present chapter. -

2,4-Dichlorophenoxyacetic Acid
2,4-Dichlorophenoxyacetic acid 2,4-Dichlorophenoxyacetic acid IUPAC (2,4-dichlorophenoxy)acetic acid name 2,4-D Other hedonal names trinoxol Identifiers CAS [94-75-7] number SMILES OC(COC1=CC=C(Cl)C=C1Cl)=O ChemSpider 1441 ID Properties Molecular C H Cl O formula 8 6 2 3 Molar mass 221.04 g mol−1 Appearance white to yellow powder Melting point 140.5 °C (413.5 K) Boiling 160 °C (0.4 mm Hg) point Solubility in 900 mg/L (25 °C) water Related compounds Related 2,4,5-T, Dichlorprop compounds Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) 2,4-Dichlorophenoxyacetic acid (2,4-D) is a common systemic herbicide used in the control of broadleaf weeds. It is the most widely used herbicide in the world, and the third most commonly used in North America.[1] 2,4-D is also an important synthetic auxin, often used in laboratories for plant research and as a supplement in plant cell culture media such as MS medium. History 2,4-D was developed during World War II by a British team at Rothamsted Experimental Station, under the leadership of Judah Hirsch Quastel, aiming to increase crop yields for a nation at war.[citation needed] When it was commercially released in 1946, it became the first successful selective herbicide and allowed for greatly enhanced weed control in wheat, maize (corn), rice, and similar cereal grass crop, because it only kills dicots, leaving behind monocots. Mechanism of herbicide action 2,4-D is a synthetic auxin, which is a class of plant growth regulators. -

2017 Spray Bulletin for Commercial Tree Fruit Growers Virginia, West Virginia, and University of Maryland Extension
Publication 456-419 2017 Spray Bulletin for Commercial Tree Fruit Growers Virginia, West Virginia, and University of Maryland Extension West Virginia University Table of Incompatibilities abamectin (Abba, Agri-Mek, Temprano) abamectin + thiamethoxam (Agri-Flex) acequinocyl (Kanemite) azadirachtin (Aza-Direct, Neemazad, Neemix) Topsin-M bifenazate (Acramite) Bt Bordeaux Mixture buprofezin (Centaur) captan carbaryl (Sevin) chlorantraniliprole (Altacor) Compatible; safe and effective chlorothalonil (Bravo) chlorpyrifos (Lorsban, Nufos, Yuma) if used as recommended. clofentezine, hexythiazox (Apollo, Onager, Savey) CM virus (Carpovirusine, Cyd-X, Madex) Not compatible; unsafe, one copper cyantraniliprole (Exirel) or both materials ineffective. cyflumetofen (Nealta) diazinon Caution - not always safe or dichloran (Botran) effective. See text. diflubenzuron (Dimilin) dinotefuran (Scorpion, Venom) dodine (Syllit) Information lacking or mixture emamectin benzoate (Proclaim) not probable. etoxazole (eal) fenarimol (Rubigan) fenbutatin (Vendex) Use wettable powder fenpyroximate (Portal) formulation only. ferbam flonicamid (Beleaf) fluazinam (Omega) flubendiamide (Belt) flubendiamide + buprofezin (Tourismo) flupyradifurone (Sivanto)) formetanate hydrochl. (Carzol) imidacloprid + beta-cyfluthrin (Leverage) indoxacarb (Avaunt) iprodione (Rovral) kaolin (Surround) lambda-cyhalothrin + chlorantraniliprole (Voliam Xpress), lambda-cyhalothrin + thiamethoxam (Endigo) lime malathion mancozeb, iram methidathion (Supracide) methomyl (Lannate) methoxyfenozide -

WO 2012/125779 Al 20 September 2012 (20.09.2012) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2012/125779 Al 20 September 2012 (20.09.2012) P O P C T (51) International Patent Classification: CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, A01N 43/90 (2006.01) DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, (21) International Application Number: KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, PCT/US20 12/029 153 MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (22) International Filing Date: OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SC, SD, 15 March 2012 (15.03.2012) SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of regional protection available): ARIPO (BW, GH, (30) Priority Data: GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, 61/453,202 16 March 201 1 (16.03.201 1) US UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, (71) Applicant (for all designated States except US) : DOW DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, AGROSCIENCES LLC [US/US]; 9330 Zionsville Road, LV, MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, Indianapolis, IN 46268 (US). -

Solventless Formulation of Triclopyr Butoxyethyl
(19) & (11) EP 1 983 829 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A01N 43/40 (2006.01) A01N 25/30 (2006.01) 13.01.2010 Bulletin 2010/02 A01N 25/04 (2006.01) A01P 13/00 (2006.01) (21) Application number: 06837962.7 (86) International application number: PCT/US2006/044751 (22) Date of filing: 17.11.2006 (87) International publication number: WO 2007/094836 (23.08.2007 Gazette 2007/34) (54) SOLVENTLESS FORMULATION OF TRICLOPYR BUTOXYETHYL ESTER LÖSUNGSMITTELFREIE FORMULIERUNG VON TRICLOPYR-BUTOXYETHYLESTER FORMULE SANS SOLVANT D’ESTER DE BUTOXYETHYLE DU TRICYCLOPYR (84) Designated Contracting States: • POMPEO, Michael, P. DE ES FR GB IT Sumter, SC 29150 (US) (30) Priority: 15.02.2006 US 773417 P (74) Representative: Weiss, Wolfgang Weickmann & Weickmann (43) Date of publication of application: Patentanwälte 29.10.2008 Bulletin 2008/44 Postfach 86 08 20 81635 München (DE) (73) Proprietors: • Dow AgroSciences LLC (56) References cited: Indianapolis, WO-A-96/28027 WO-A-2004/093546 Indiana 46268-1054 (US) US-A1- 5 466 659 • Jensen, Jeffrey, Lee Brownsburg IN 46112 (US) • BOVEY RODNEY W ET AL: "Honey mesquite (Prosopis glandulosa) control by synergistic (72) Inventors: action of clopyralid: Triclopyr mixtures" WEED • JENSEN, Jeffrey, Lee SCIENCE, vol. 40, no. 4, 1992, pages 563-567, Brownsburg, IN 46112 (US) XP009080681 ISSN: 0043-1745 • KLINE, William, N., III • BEBAWI F F ET AL: "Impact of foliar herbicides Duluth, GA 30096 (US) on pod and seed behaviour of rust-infected • BURCH, Patrick, L. rubber vine (Cryptostegia grandiflora) plants" Christiansburg, VA 24073 (US) PLANT PROTECTION QUARTERLY, vol. -

Oecd Guideline for the Testing of Chemicals
Paris 28 November 2008 [email protected] OECD GUIDELINE FOR THE TESTING OF CHEMICALS DRAFT PROPOSAL FOR A NEW GUIDELINE: 436 Acute Inhalation Toxicity - Acute Toxic Class (ATC) Method INTRODUCTION 1. OECD Guidelines for the Testing of Chemicals are periodically reviewed in the light of scientific progress, changing regulatory needs and animal welfare considerations. The first acute inhalation Test Guideline 403 was adopted in 1981, and has since been revised (1). Development of an Inhalation Acute Toxic Class (ATC) method (2)(3)(4) was considered appropriate following the adoption of the revised oral ATC method (TG 423)(5) in December 2001. A retrospective validation study of the acute toxic class testing method for acute inhalation toxicity showed that the method is suitable for being used for Classification and Labelling purposes (6). The inhalation ATC Guideline will allow the use of serial steps of fixed target concentrations to provide a ranking of test article toxicity. Lethality is used as key endpoint. However, animals in severe pain or distress, suffering or impending death should be humanely killed to minimize suffering. Guidance on humane endpoints is available in the OECD Guidance Document No. 19 (7). 2. Guidance on the conduct and interpretation of the TG 436 can be found in the Guidance Document on Acute Inhalation Toxicity Testing (8). 3. Definitions used in the context of this Guideline are provided in Annex 1. 4. The method provides information on the hazardous properties and allows the substance to be ranked and classified according to the United Nations (UN) Globally Harmonized System of Classification and Labelling of Chemicals (GHS) for the classification of chemicals that cause acute toxicity (9). -

Integrated Pest Management Strategies
Table of Contents Introduction ................................................................................................................................................. 3 Responsibilities ........................................................................................................................................... 4 IPM Coordinator .......................................................................................................................................... 4 Pesticide Applications ................................................................................................................................ 6 Inspection and Monitoring ......................................................................................................................... 6 Integrated Pest Management Strategies ................................................................................................... 7 Exterior Entrance Points ............................................................................................................................ 7 Classrooms and Offices ............................................................................................................................ 7 Cafeterias and Kitchens ............................................................................................................................ 8 Restrooms ................................................................................................................................................. 9 Outside Areas -

High Lethality and Minimal Variation After Acute Self-Poisoning with Carbamate Insecticides in Sri Lanka – Implications for Global Suicide Prevention
Clinical Toxicology ISSN: 1556-3650 (Print) 1556-9519 (Online) Journal homepage: http://www.tandfonline.com/loi/ictx20 High lethality and minimal variation after acute self-poisoning with carbamate insecticides in Sri Lanka – implications for global suicide prevention Thomas Lamb, Liza R. Selvarajah, Fahim Mohamed, Shaluka Jayamanne, Indika Gawarammana, Ahmed Mostafa, Nicholas A. Buckley, Michael S. Roberts & Michael Eddleston To cite this article: Thomas Lamb, Liza R. Selvarajah, Fahim Mohamed, Shaluka Jayamanne, Indika Gawarammana, Ahmed Mostafa, Nicholas A. Buckley, Michael S. Roberts & Michael Eddleston (2016): High lethality and minimal variation after acute self-poisoning with carbamate insecticides in Sri Lanka – implications for global suicide prevention, Clinical Toxicology To link to this article: http://dx.doi.org/10.1080/15563650.2016.1187735 © 2016 The Authors. Published by Informa UK Limited, trading as Taylor & Francis Group. Published online: 02 Jun 2016. Submit your article to this journal View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=ictx20 Download by: [The University of Edinburgh] Date: 02 June 2016, At: 05:24 CLINICAL TOXICOLOGY, 2016 http://dx.doi.org/10.1080/15563650.2016.1187735 CLINICAL RESEARCH High lethality and minimal variation after acute self-poisoning with carbamate insecticides in Sri Lanka – implications for global suicide prevention Thomas Lamba, Liza R. Selvarajaha, Fahim Mohamedb,c, -

China Releases New Maximum Residue Limits for Pesticides In
GB 2763-2016 THIS REPORT CONTAINS ASSESSMENTS OF COMMODITY AND TRADE ISSUES MADE BY USDA STAFF AND NOT NECESSARILY STATEMENTS OF OFFICIAL U.S. GOVERNMENT POLICY Voluntary - Public Date: 3/31/2017 GAIN Report Number: CH17016 China - Peoples Republic of Post: Beijing China Releases New Maximum Residue Limits for Pesticides in Food Report Categories: FAIRS Subject Report Approved By: Lisa Anderson Prepared By: FAS Staff Report Highlights: On December 18, 2016, the Chinese National Health and Family Planning Commission, Ministry of Agriculture, China Food and Drug Administration released the National Food Safety Standard - Maximum Residue Limits for Pesticides in Foods (GB 2763-2016). The standard will replace the current MRL Standard (GB 2763-2014) and will be implemented on June 18, 2017. This report provides an unofficial translation of the standard. Editors’ Note: The asterisk appearing in the MRL column means that the limit is a temporary MRL. A temporary MRL is usually set under the following four conditions: 1. The dietary risk assessment data is incomplete; 2. The Acceptable Daily Intake (ADI) is temporary (ADI is used as the basis for MRL setting); 3. There is no surveillance or analysis method for the MRL that complies with the standard requirements; 4. In emergency situations, the pesticide is approved to be used on un-registered crops. I GB 2763-2016 General Information: BEGIN TRANSLATION ICS 65.100 G 25 GB National Standard of the People’s Republic of China GB 2763—2016 Replacing GB 2763 - 2014 National food safety standard Maximum Residue Limits for Pesticides in Food General Information: National Health and Family Planning Commission Issued by: Ministry of Agriculture China Food and Drug Administration Issued on: 2016-12-18 Implementation:2017-06-18 II GB 2763-2016 Table of Content Preface ............................................................................................................................................................... -

State of the Art Report on Mixture Toxicity
State of the Art Report on Mixture Toxicity Final Report Executive Summary 22 December 2009 Study Contract Number 070307/2007/485103/ETU/D.1 Contractor The School of Pharmacy University of London (ULSOP) Sub-Contractors Göteborg University (UGOT) Faust & Backhaus Environmental Consulting GbR (FBEC) Responsible Scientists Prof. Dr. Andreas Kortenkamp (ULSOP) Assoc.-Prof. Dr. Thomas Backhaus (UGOT) Dr. Michael Faust (FBEC) 1 Disclaimer: Please note that this document was produced by a contractor in fulfillment of study contract No. 070307/2007/485103/ETU/D.1. The views expressed in the document are those of the contractor and do not represent the position of the Commission services. The Commission services do not accept any liability with regard to the contents of this document. State of the Art Report on Mixture Toxicity – Final Report, Executive Summary Table of contents 1. Terms of reference, scope, definitions ..................................................................... 3 2. The scientific state of the art of mixture toxicology ................................................. 5 3. The regulatory state of the art of mixture toxicology ............................................. 13 4. Recommendations................................................................................................... 17 2 State of the Art Report on Mixture Toxicity – Final Report, Executive Summary Executive Summary This report details the findings of a project on mixture toxicology and ecotoxicology commissioned by the European Commission, DG -

MATERIAL SAFETY DATA SHEET Issued By: Arysta Lifescience South Africa Phone: 031 514 5600 Poisons Helpline 0861 555 777 Spillage Helpline (Spill Tech) 086 100 0366
Product Name: Mitrus Page 1 of 5 SECTION 1 - PRODUCT & COMPANY IDENTIFICATION ARYSTA LifeScience South Africa (Pty) Ltd Tel: 031 514 5600 Co. Reg. No.: 2009/019713/07 Fax: 031 514 5611 7 Sunbury Office Park, Off Douglas Saunders Drive, La Lucia Ridge, South Africa, 4019 e-mail: [email protected] Web address: arystalifescience.co.za Substance: fenbutatin oxide (550 g/ℓ) Product Name: MITRUS Product Use: Insecticide Creation Date: August 2006 Revision Date: August 2019 24 Hr Emergency Number: In case of Poisoning: Poisons Helpline 0861 555 777 In case of Spillage: Spill Tech Oil & Chemical Pollution Control 086 100 0366 / 083 253 6618 SECTION 2 - COMPOSITION / INFORMATION ON INGREDIENTS Common name: FENBUTATIN OXIDE (550 g/ℓ) Chemical Name: Bis[tris(2-methyl-2-phenylpropyl)tin]oxide CAS No.: 13356-08-6 Chemical Family: Organotin Use: Contact acaricide for the control of mites. Formulation: Suspension concentrate Symbols: Xi, N Risk-phrase(s): R20/22, R36/37/38, R40, R43, R50/53 SECTION 3 - HAZARD IDENTIFICATION Toxicity class: Of low toxicity order. Slightly Toxic. Eye contact: May cause irritation. Causing redness, pain and blurred vision. Skin contact: Moderate irritation. Skin sensitization reaction may occur in some individuals. Avoid long-term contact with the material. Ingestion: Harmful. Large amounts ingested may cause nausea, vomiting, diarrhea, convulsions, incoordination, fatigue, hypothermia and respiratory paralysis. Neurotoxicity may be referable to carbon disulfide released in the acid environment of the stomach. Inhalation: Slightly toxic. Inhalation may cause mild inflammation and irritation of the respiratory tract. May cause sore throat, coughing, sneezing, shortness of breath, dizziness and dullness. -
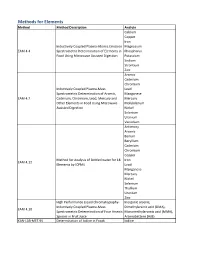
Method Description
Methods for Elements Method Method Description Analyte Calcium Copper Iron Inductively Coupled Plasma-Atomic Emission Magnesium EAM 4.4 Spectrometric Determination of Elements in Phosphorus Food Using Microwave Assisted Digestion Potassium Sodium Strontium Zinc Arsenic Cadmium Chromium Inductively Coupled Plasma-Mass Lead Spectrometric Determination of Arsenic, Manganese EAM 4.7 Cadmium, Chromium, Lead, Mercury and Mercury Other Elements in Food Using Microwave Molybdenum Assisted Digestion Nickel Selenium Uranium Vanadium Antimony Arsenic Barium Beryllium Cadmium Chromium Copper Method for Analysis of Bottled water for 18 Iron EAM 4.12 Elements by ICPMS Lead Manganese Mercury Nickel Selenium Thallium Uranium Zinc High Performance Liquid Chromatography- Inorganic arsenic, Inductively Coupled Plasma-Mass Dimethylarsinic acid (DMA), EAM 4.10 Spectrometric Determination of Four Arsenic Monomethylarsonic acid (MMA), Species in Fruit Juice Arsenobetaine (AsB) KAN-LAB-MET.95 Determination of Iodine in Foods Iodine Methods for Radionuclides Method Method Description Analyte Determination of Strontium-90 in Foods by WEAC.RN.METHOD.2.0 Strontium-90 Internal Gas-Flow Proportional Counting Americium-241 Cesium-134 Cesium-137 Determination of Gamma-Ray Emitting Cobalt-60 WEAC.RN.METHOD.3.0 Radionuclides in Foods by High-Purity Potassium-40 Germanium Spectrometry Radium-226 Ruthenium-103 Ruthenium-106 Thorium-232 Methods for Pesticides/Industrial Chemicals Method Method Description Analyte Extraction Method: Analysis of Pesticides KAN-LAB-PES.53 and