The Role of Angiomotin in Endothelial Cell Motility and Cell-Cell Junction Formation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Machine-Learning and Chemicogenomics Approach Defi Nes and Predicts Cross-Talk of Hippo and MAPK Pathways
Published OnlineFirst November 18, 2020; DOI: 10.1158/2159-8290.CD-20-0706 RESEARCH ARTICLE Machine -Learning and Chemicogenomics Approach Defi nes and Predicts Cross-Talk of Hippo and MAPK Pathways Trang H. Pham 1 , Thijs J. Hagenbeek 1 , Ho-June Lee 1 , Jason Li 2 , Christopher M. Rose 3 , Eva Lin 1 , Mamie Yu 1 , Scott E. Martin1 , Robert Piskol 2 , Jennifer A. Lacap 4 , Deepak Sampath 4 , Victoria C. Pham 3 , Zora Modrusan 5 , Jennie R. Lill3 , Christiaan Klijn 2 , Shiva Malek 1 , Matthew T. Chang 2 , and Anwesha Dey 1 ABSTRACT Hippo pathway dysregulation occurs in multiple cancers through genetic and non- genetic alterations, resulting in translocation of YAP to the nucleus and activation of the TEAD family of transcription factors. Unlike other oncogenic pathways such as RAS, defi ning tumors that are Hippo pathway–dependent is far more complex due to the lack of hotspot genetic alterations. Here, we developed a machine-learning framework to identify a robust, cancer type–agnostic gene expression signature to quantitate Hippo pathway activity and cross-talk as well as predict YAP/TEAD dependency across cancers. Further, through chemical genetic interaction screens and multiomics analyses, we discover a direct interaction between MAPK signaling and TEAD stability such that knockdown of YAP combined with MEK inhibition results in robust inhibition of tumor cell growth in Hippo dysregulated tumors. This multifaceted approach underscores how computational models combined with experimental studies can inform precision medicine approaches including predictive diagnostics and combination strategies. SIGNIFICANCE: An integrated chemicogenomics strategy was developed to identify a lineage- independent signature for the Hippo pathway in cancers. -

Feeling the Force: Role of Amotl2 in Normal Development and Cancer
Department of Oncology and Pathology Karolinska Institute, Stockholm, Sweden FEELING THE FORCE: ROLE OF AMOTL2 IN NORMAL DEVELOPMENT AND CANCER Aravindh Subramani Stockholm 2019 All previously published papers were reproduced with permission from the publisher. Front cover was modified from ©Renaud Chabrier and illustrated by Yu-Hsuan Hsu Published by Karolinska Institute. Printed by: US-AB Stockholm © Aravindh Subramani, 2019 ISBN 978-91-7831-426-3 Feeling the force: Role of AmotL2 in normal development and cancer. THESIS FOR DOCTORAL DEGREE (Ph.D.) J3:04, Torsten N Wiesel, U410033310, Bioclinicum, Karolinska University Hospital, Solna, Stockholm Friday, April 12th, 2019 at 13:00 By Aravindh Subramani Principal Supervisor: Opponent: Professor. Lars Holmgren Professor. Marius Sudol Karolinska Institute National University of Singapore Department of Oncology-Pathology Mechanobiology Institute (MBI) Co-supervisor(s): Examination Board: Docent. Jonas Fuxe Docent. Kaisa Lehti Karolinska Institute Karolinska institute Department of Microbiology, Tumor and Cell Department of Microbiology and Tumor Biology Biology (MTC) center (MTC) Associate Professor. Johan Hartman Docent. Ingvar Ferby Karolinska Institute Uppsala University Department of Oncology-Pathology Department of Medical Biochemistry and Microbiology Docent. Mimmi Shoshan Karolinska Institute Department of Oncology-Pathology To my mom and dad “Real knowledge is to know the extent of one's ignorance.” (Confucius) ABSTRACT Cells that fabricate the body, dwell in a very heterogeneous environment. Self-organization of individual cells into complex tissues and organs at the time of growth and revival is brought about by the combinatory action of biomechanical and biochemical signaling processes. Tissue generation and functional organogenesis, requires distinct cell types to unite together and associate with their corresponding microenvironment in a spatio-temporal manner. -

The Role of the C-Terminus Merlin in Its Tumor Suppressor Function Vinay Mandati
The role of the C-terminus Merlin in its tumor suppressor function Vinay Mandati To cite this version: Vinay Mandati. The role of the C-terminus Merlin in its tumor suppressor function. Agricultural sciences. Université Paris Sud - Paris XI, 2013. English. NNT : 2013PA112140. tel-01124131 HAL Id: tel-01124131 https://tel.archives-ouvertes.fr/tel-01124131 Submitted on 19 Mar 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 TABLE OF CONTENTS Abbreviations ……………………………………………………………………………...... 8 Resume …………………………………………………………………………………… 10 Abstract …………………………………………………………………………………….. 11 1. Introduction ………………………………………………………………………………12 1.1 Neurofibromatoses ……………………………………………………………………….14 1.2 NF2 disease ………………………………………………………………………………15 1.3 The NF2 gene …………………………………………………………………………….17 1.4 Mutational spectrum of NF2 gene ………………………………………………………..18 1.5 NF2 in other cancers ……………………………………………………………………...20 2. ERM proteins and Merlin ……………………………………………………………….21 2.1 ERMs ……………………………………………………………………………………..21 2.1.1 Band 4.1 Proteins and ERMs …………………………………………………………...21 2.1.2 ERMs structure ………………………………………………………………………....23 2.1.3 Sub-cellular localization and tissue distribution of ERMs ……………………………..25 2.1.4 ERM proteins and their binding partners ……………………………………………….25 2.1.5 Assimilation of ERMs into signaling pathways ………………………………………...26 2.1.5. A. ERMs and Ras signaling …………………………………………………...26 2.1.5. B. ERMs in membrane transport ………………………………………………29 2.1.6 ERM functions in metastasis …………………………………………………………...30 2.1.7 Regulation of ERM proteins activity …………………………………………………...31 2.1.7. -

Chapter 8 ANGIOSTATIN Generation, Structure and Function of the Isoforms
Chapter 8 ANGIOSTATIN Generation, Structure and Function of the Isoforms Jennifer A. Doll and Gerald A. Soff Northwestern University Feinberg School of Medicine, Department of Medicine, Division oj Hernatology/Oncology and the Robert H. Lurie Comprehensive Cancer Center, Chicago, ZL 1. INTRODUCTION: DISCOVERY AND FUNCTION OF ANGIOSTATIN The discovery of angiostatin in 1994 provided a major advance in the field of angiogenesis research. As Folkman first proposed in 1971, angiogenesis, the growth of new vessels from the pre-existing vasculature, is required for tumors to grow beyond a few millimeters in diameter and for tumor metastasis'. With a few exceptions (wound healing and reproductive cycles), the vasculature in the adult is maintained in a quiescent state by a net balance of angiogenic inducers and inhibitors secreted into the tissue Tumors shift this balance to favor vessel growth, by increasing inducer levels, decreasing inhibitor levels, or most often, a combination of Tumor angiogenesis involves many processes, including increased vascular permeability, endothelial cell activation, proliferation, migration and tube formation as well as matrix degradation. Designing therapies targeting any of these steps would inhibit angiogenesis, and thus inhibit tumor growth. Therefore, much research has been devoted to developing such agents for use in cancer therapy. Numerous inducers and inhibitors of angiogenesis have now been identified, both endogenous and exogenous. It is of interest to note that the endogenous regulators, both inducers and inhibitors, span extremely diverse groups of molecules, including growth factors and cytokines, proteins and enzymes, protein cleavage products, enzyme inhibitors, carbohydrates, lipids, hormones and Most of the naturally occurring angiogenesis inhibitors, such as thrombospondin-1 and pigment epithelium- 176 CYTOKINES AND CANCER derived factor, have a wide variety of cellular activities and affect multiple cell types6,'. -

TEAD4/YAP1/WWTR1 Prevent the Premature Onset of Pluripotency
bioRxiv preprint doi: https://doi.org/10.1101/663005; this version posted June 6, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 TEAD4/YAP1/WWTR1 prevent the premature onset of pluripotency prior to the 16- 2 cell stage 3 4 Tristan Frum1, Jennifer Watts2,3, and Amy Ralston1,3# 5 6 1Department of Biochemistry and Molecular Biology, Michigan State University, East 7 Lansing, Michigan, 48824, United States. 8 9 2Physiology Graduate Program, Michigan State University, East Lansing, Michigan, 10 48824, United States. 11 12 3Reproductive and Developmental Biology Training Program, Michigan State University, 13 East Lansing, Michigan, 48824, United States. 14 15 #) Correspondence: [email protected] 16 17 Running title: Mechanism preventing premature Sox2 18 19 Key words: preimplantation, stem cell progenitors, HIPPO signaling 20 1 bioRxiv preprint doi: https://doi.org/10.1101/663005; this version posted June 6, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 21 Abstract 22 In the mouse embryo, pluripotent cells arise inside the embryo around the 16-cell stage. 23 During these early stages, Sox2 is the only gene whose expression is known to be 24 induced specifically within inside cells as they are established. -

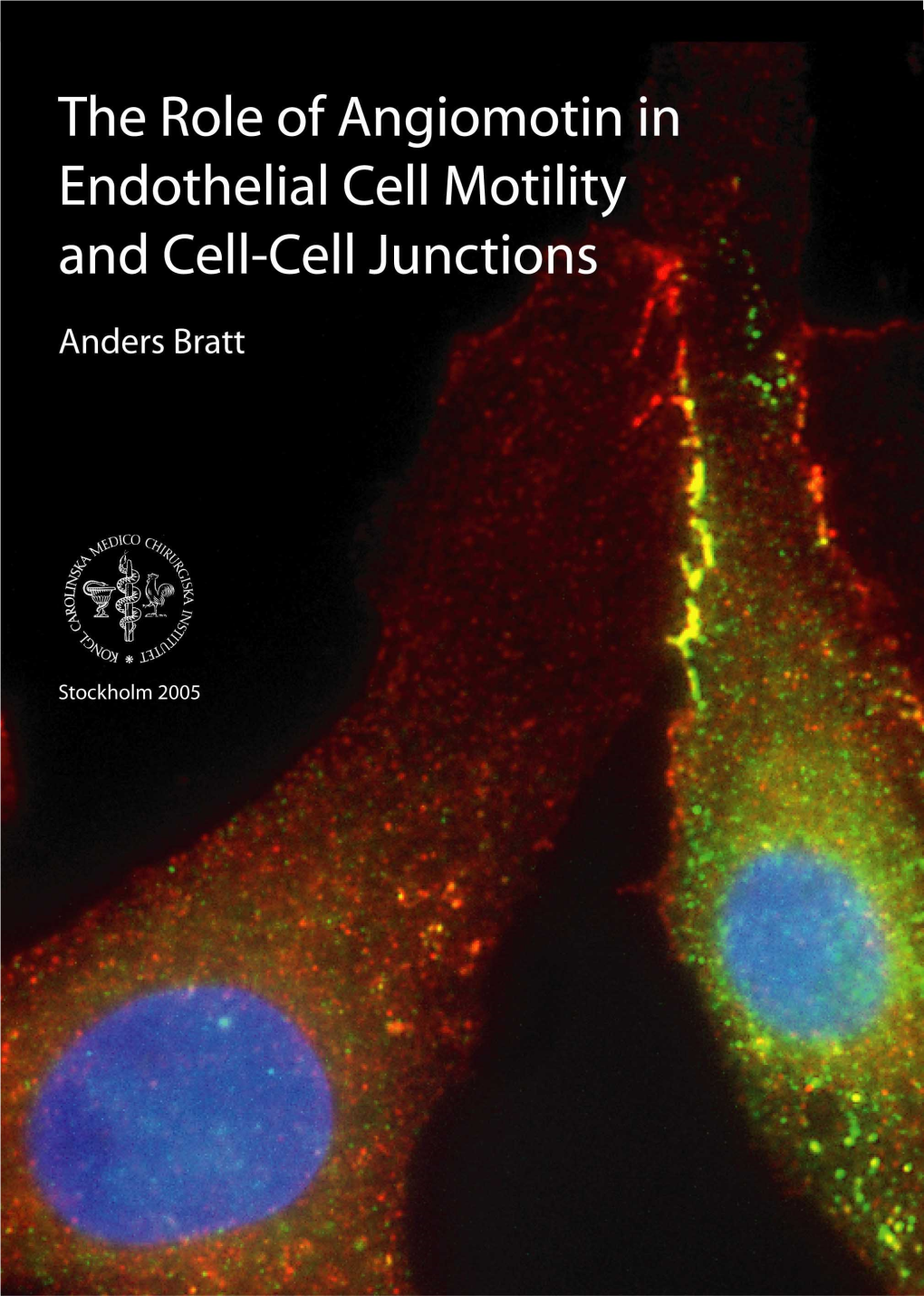
Making Endothelial Cells Move – a Study of Angiomotin and Binding Partners
MAKING ENDOTHELIAL CELLS MOVE – A STUDY OF ANGIOMOTIN AND BINDING PARTNERS From the Department of Oncology and Pathology Karolinska Institutet, Stockholm, Sweden MAKING ENDOTHELIAL CELLS MOVE – A STUDY OF ANGIOMOTIN AND BINDING PARTNERS Nathalie Luna Persson Stockholm 2012 MAKING ENDOTHELIAL CELLS MOVE – A STUDY OF ANGIOMOTIN AND BINDING PARTNERS All previously published papers were reproduced with permission from the publisher. Published by Karolinska Institutet. Printed by Larserics Digital Print AB. © Nathalie Luna Persson, 2012. Cover photo: Blood vessels invading into a bFGF-stimulated matrigel plug. Red: Blood vessel (CD31). Green: Pericytes (NG2). Staining and design by Nathalie Luna Persson. Microscopy by Sara Hultin. ISBN 978-91-7457-671-9 MAKING ENDOTHELIAL CELLS MOVE – A STUDY OF ANGIOMOTIN AND BINDING PARTNERS MAKING ENDOTHELIAL CELLS MOVE – A STUDY OF ANGIOMOTIN AND BINDING PARTNERS Populärvetenskaplig sammanfattning Blodkärl finns i hela kroppen. De syns för det mesta som turkosa strängar under huden när vi ser efter. De är oerhört viktiga för vår överlevnad och bildandet av nya blodkärl är som allra viktigast när våra sår läker, under menstruation och graviditet samt självklart under fosterutvecklingen i mammans mage. Lagom är bäst tycker kroppen och därför ställer överdriven eller otillräcklig blodtillförsel till med problem och sjukdom. I den här avhandlingen har jag fokuserat på sjukdomen cancer. Tumörer kan inte överleva och växa bortom ett knappnålshuvuds storlek utan syre och näring. Därför åker de snålskjuts på kroppens eget maskineri och lockar till sig blodkärl. I och med detta kan tumören inte bara växa utan har också införskaffat ett sätt att sprida sig på, metastasera. Sammantaget är detta anledningen till att hög blodkärlsnärvaro i tumörer inte är bra när man talar om patienters prognos. -

Serum Deprivation Inhibits the Transcriptional Co-Activator YAP And
Serum deprivation inhibits the transcriptional co-activator YAP and cell growth via phosphorylation of the 130-kDa isoform of Angiomotin by the LATS1/2 protein kinases Jacob J. Adlera, Derrick E. Johnsona, Brigitte L. Hellera, Lauren R. Bringmana, William P. Ranahana, Michael D. Conwellb, Yang Sunb, Andy Hudmona,c, and Clark D. Wellsa,1 aDepartment of Biochemistry and Molecular Biology, bDepartment of Ophthalmology, Glick Eye Institute, and cStark Neuroscience Research Institute, Indiana University School of Medicine, Indianapolis, IN 46202 Edited* by Tony Pawson, Samuel Lunenfeld Research Institute, Toronto, ON, Canada, and approved September 17, 2013 (received for review May 3, 2013) Large tumor suppressor (LATS)1/2 protein kinases transmit Hippo all bind and inhibit YAP and TAZ (17–19). Amot associates with signaling in response to intercellular contacts and serum levels to cell junctions and binds apical polarity proteins, which underlie its limit cell growth via the inhibition of Yes-associated protein (YAP). ability to control cell shape and migration (20–23). The 130 kDa Here low serum and high LATS1 activity are found to enhance the isoform of Amot (Amot130), unlike the 80-kDa isoform (Amot80) levels of the 130-kDa isoform of angiomotin (Amot130) through that promotes cell growth (24), binds and inhibits YAP through phosphorylation by LATS1/2 at serine 175, which then forms a cytosolic sequestration (17, 18) and by facilitating its degradation binding site for 14-3-3. Such phosphorylation, in turn, enables the (25) in a manner that can be independent of YAP phosphorylation ubiquitin ligase atrophin-1 interacting protein (AIP)4 to bind, ubiq- by LATS1/2 at residue Ser-127 (17, 18). -

AMOT Suppresses Tumor Progression Via Regulating DNA Damage Response Signaling in Diffuse Large B-Cell Lymphoma
Cancer Gene Therapy https://doi.org/10.1038/s41417-020-00258-5 ARTICLE AMOT suppresses tumor progression via regulating DNA damage response signaling in diffuse large B-cell lymphoma 1,2,3 1,2 1,2 1,2 1,2 1,2,4,5,6 1,2,4,5,6 Tan Sang ● Juan Yang ● Jiarui Liu ● Yang Han ● Ying Li ● Xiangxiang Zhou ● Xin Wang Received: 7 June 2020 / Revised: 22 October 2020 / Accepted: 4 November 2020 © The Author(s), under exclusive licence to Springer Nature America, Inc. 2021 Abstract Angiomotin (AMOT) is a membrane protein that is aberrantly expressed in a variety of solid tumors. Accumulating evidence support that AMOT is involved in the pathological processes of tumor proliferation, apoptosis, and invasion. However, the potential role of AMOT in the pathogenesis of diffuse large B-cell lymphoma (DLBCL) remains elusive. In the present study, we investigated the expression level and biological function of AMOT in DLBCL. AMOT expression was significantly reduced in DLBCL biopsy section, and low AMOT expression was associated with poor clinical prognosis. Overexpression of AMOT by lentivirus in human DLBCL cells induced cell viability inhibition concomitant with an increased percentage of cells in G1 phase and decreased percentage in S phase. Moreover, AMOT upregulation increased the 1234567890();,: 1234567890();,: sensitivity of DLBCL cells to doxorubicin. Furthermore, overexpression of AMOT led to reduced activation of key kinases for the DNA damage response (DDR). The above results indicated that AMOT acts as a tumor suppressor via inhibition of the DDR, thus reducing the viability while increasing the chemosensitivity in DLBCL. -

Early Prediction of Revascularisation by Angiomotin-Targeting Positron
Early prediction of revascularisation by angiomotin-targeting positron emission tomography Anaïs Moyon, Philippe Garrigue, Laure Balasse, Samantha Fernandez, Pauline Brige, Marie Nollet, Guillaume Hache, Marcel Blot-Chabaud, Francoise Dignat-George, Benjamin Guillet To cite this version: Anaïs Moyon, Philippe Garrigue, Laure Balasse, Samantha Fernandez, Pauline Brige, et al.. Early prediction of revascularisation by angiomotin-targeting positron emission tomography. Theranostics, Ivyspring International Publisher, 2018, 8 (18), pp.4985-4994. 10.7150/thno.27728. hal-02060575 HAL Id: hal-02060575 https://hal-amu.archives-ouvertes.fr/hal-02060575 Submitted on 26 May 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Theranostics 2018, Vol. 8, Issue 18 4985 Ivyspring International Publisher Theranostics 2018; 8(18): 4985-4994. doi: 10.7150/thno.27728 Research Paper Early prediction of revascularisation by angiomotin- targeting positron emission tomography Anais Moyon1,2,3; Philippe Garrigue1,2,3; Laure Balasse2; Samantha Fernandez2; Pauline Brige2; Marie Nollet1; Guillaume Hache1,2; Marcel Blot-Chabaud1; Françoise Dignat-George1, 4; Benjamin Guillet1,2,3 1. Aix Marseille Univ, INSERM 1263, INRA 1260, C2VN, Marseille, France. 2. Aix-Marseille Univ, CERIMED, Marseille, France. 3. Service de Radiopharmacie, APHM, Marseille, France. -

Exploiting the Use of Plasminogen Kringle Domains for Cancer Gene Therapy
EXPLOITING THE USE OF PLASMINOGEN KRINGLE DOMAINS FOR CANCER GENE THERAPY Sabrina R. Perri Division of Experimental Medicine, Deparlment of Medicine Faculty of Medicine, McGiII University Montreal, Quebec, Canada February 2007 A thesis submitled to the Faculty of Graduate Studies and Research in partial fulfillment of the requirements of the degree of Doctor of Philosophy (Ph. O.). ©2007 Sabrina R. Perri Library and Bibliothèque et 1+1 Archives Canada Archives Canada Published Heritage Direction du Branch Patrimoine de l'édition 395 Wellington Street 395, rue Wellington Ottawa ON K1A ON4 Ottawa ON K1A ON4 Canada Canada Your file Votre référence ISBN: 978-0-494-32376-2 Our file Notre référence ISBN: 978-0-494-32376-2 NOTICE: AVIS: The author has granted a non L'auteur a accordé une licence non exclusive exclusive license allowing Library permettant à la Bibliothèque et Archives and Archives Canada to reproduce, Canada de reproduire, publier, archiver, publish, archive, preserve, conserve, sauvegarder, conserver, transmettre au public communicate to the public by par télécommunication ou par l'Internet, prêter, telecommunication or on the Internet, distribuer et vendre des thèses partout dans loan, distribute and sell th es es le monde, à des fins commerciales ou autres, worldwide, for commercial or non sur support microforme, papier, électronique commercial purposes, in microform, et/ou autres formats. paper, electronic and/or any other formats. The author retains copyright L'auteur conserve la propriété du droit d'auteur ownership and moral rights in et des droits moraux qui protège cette thèse. this thesis. Neither the thesis Ni la thèse ni des extraits substantiels de nor substantial extracts from it celle-ci ne doivent être imprimés ou autrement may be printed or otherwise reproduits sans son autorisation. -

Functional Relationship Between Merlin and the ERM Proteins
Functional Relationship between Merlin and the ERM Proteins The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Hebert, Alan. 2012. Functional Relationship between Merlin and the ERM Proteins. Doctoral dissertation, Harvard University. Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:9909639 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA © 2012 – Alan M. Hebert All rights reserved. Advisor: Dr. Andrea I. McClatchey Alan M. Hebert Functional Relationship Between Merlin and the ERM Proteins Abstract The ability to spatially restrict specific activities across the cell cortex functionally defines individual cells and tissues. This is achieved, in part, via the assembly of protein complexes that link the plasma membrane to the underlying cortical actin cytoskeleton. The neurofibromatosis type 2 (NF2) tumor suppressor Merlin and closely related ERM proteins (Ezrin, Radixin and Moesin) are a special class of such membrane:cytoskeleton associated proteins that function to organize specialized cortical domains. In addition to their high degree of similarity, mounting evidence suggests that Merlin/ERMs share a functional relationship, which is largely unexplored. Unlike Merlin, the ERMs are not known to inhibit cell proliferation; in fact, Ezrin is thought to promote tumor metastasis. Defining the relationship between Merlin and the ERMs is essential to appreciating their respective roles in cancer development. Here I demonstrate a novel role for Merlin and the ERMs in generating cortical asymmetry in the absence of external cues. -

Hippo Signaling Pathway in Gliomas
cells Review Hippo Signaling Pathway in Gliomas Konstantin Masliantsev 1,2,3 , Lucie Karayan-Tapon 1,2,3 and Pierre-Olivier Guichet 1,2,3,* 1 Inserm U1084, Laboratoire de Neurosciences Expérimentales et Cliniques, F-86073 Poitiers, France; [email protected] (K.M.); [email protected] (L.K.-T.) 2 Université de Poitiers, F-86073 Poitiers, France 3 CHU de Poitiers, Laboratoire de Cancérologie Biologique, F-86022 Poitiers, France * Correspondence: [email protected] Abstract: The Hippo signaling pathway is a highly conserved pathway involved in tissue devel- opment and regeneration that controls organ size through the regulation of cell proliferation and apoptosis. The core Hippo pathway is composed of a block of kinases, MST1/2 (Mammalian STE20-like protein kinase 1/2) and LATS1/2 (Large tumor suppressor 1/2), which inhibits nuclear translocation of YAP/TAZ (Yes-Associated Protein 1/Transcriptional co-activator with PDZ-binding motif) and its downstream association with the TEAD (TEA domain) family of transcription factors. This pathway was recently shown to be involved in tumorigenesis and metastasis in several cancers such as lung, breast, or colorectal cancers but is still poorly investigated in brain tumors. Gliomas are the most common and the most lethal primary brain tumors representing about 80% of malignant central nervous system neoplasms. Despite intensive clinical protocol, the prognosis for patients remains very poor due to systematic relapse and treatment failure. Growing evidence demonstrating the role of Hippo signaling in cancer biology and the lack of efficient treatments for malignant gliomas support the idea that this pathway could represent a potential target paving the way for alternative therapeutics.