538.Full-Text.Pdf
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Classification Decisions Taken by the Harmonized System Committee from the 47Th to 60Th Sessions (2011
CLASSIFICATION DECISIONS TAKEN BY THE HARMONIZED SYSTEM COMMITTEE FROM THE 47TH TO 60TH SESSIONS (2011 - 2018) WORLD CUSTOMS ORGANIZATION Rue du Marché 30 B-1210 Brussels Belgium November 2011 Copyright © 2011 World Customs Organization. All rights reserved. Requests and inquiries concerning translation, reproduction and adaptation rights should be addressed to [email protected]. D/2011/0448/25 The following list contains the classification decisions (other than those subject to a reservation) taken by the Harmonized System Committee ( 47th Session – March 2011) on specific products, together with their related Harmonized System code numbers and, in certain cases, the classification rationale. Advice Parties seeking to import or export merchandise covered by a decision are advised to verify the implementation of the decision by the importing or exporting country, as the case may be. HS codes Classification No Product description Classification considered rationale 1. Preparation, in the form of a powder, consisting of 92 % sugar, 6 % 2106.90 GRIs 1 and 6 black currant powder, anticaking agent, citric acid and black currant flavouring, put up for retail sale in 32-gram sachets, intended to be consumed as a beverage after mixing with hot water. 2. Vanutide cridificar (INN List 100). 3002.20 3. Certain INN products. Chapters 28, 29 (See “INN List 101” at the end of this publication.) and 30 4. Certain INN products. Chapters 13, 29 (See “INN List 102” at the end of this publication.) and 30 5. Certain INN products. Chapters 28, 29, (See “INN List 103” at the end of this publication.) 30, 35 and 39 6. Re-classification of INN products. -

Whither the Keiretsu, Japan's Business Networks? How Were They Structured? What Did They Do? Why Are They Gone?
IRLE IRLE WORKING PAPER #188-09 September 2009 Whither the Keiretsu, Japan's Business Networks? How Were They Structured? What Did They Do? Why Are They Gone? James R. Lincoln, Masahiro Shimotani Cite as: James R. Lincoln, Masahiro Shimotani. (2009). “Whither the Keiretsu, Japan's Business Networks? How Were They Structured? What Did They Do? Why Are They Gone?” IRLE Working Paper No. 188-09. http://irle.berkeley.edu/workingpapers/188-09.pdf irle.berkeley.edu/workingpapers Institute for Research on Labor and Employment Institute for Research on Labor and Employment Working Paper Series (University of California, Berkeley) Year Paper iirwps-- Whither the Keiretsu, Japan’s Business Networks? How Were They Structured? What Did They Do? Why Are They Gone? James R. Lincoln Masahiro Shimotani University of California, Berkeley Fukui Prefectural University This paper is posted at the eScholarship Repository, University of California. http://repositories.cdlib.org/iir/iirwps/iirwps-188-09 Copyright c 2009 by the authors. WHITHER THE KEIRETSU, JAPAN’S BUSINESS NETWORKS? How were they structured? What did they do? Why are they gone? James R. Lincoln Walter A. Haas School of Business University of California, Berkeley Berkeley, CA 94720 USA ([email protected]) Masahiro Shimotani Faculty of Economics Fukui Prefectural University Fukui City, Japan ([email protected]) 1 INTRODUCTION The title of this volume and the papers that fill it concern business “groups,” a term suggesting an identifiable collection of actors (here, firms) within a clear-cut boundary. The Japanese keiretsu have been described in similar terms, yet compared to business groups in other countries the postwar keiretsu warrant the “group” label least. -
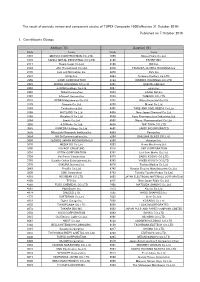
Published on 7 October 2016 1. Constituents Change the Result Of
The result of periodic review and component stocks of TOPIX Composite 1500(effective 31 October 2016) Published on 7 October 2016 1. Constituents Change Addition( 70 ) Deletion( 60 ) Code Issue Code Issue 1810 MATSUI CONSTRUCTION CO.,LTD. 1868 Mitsui Home Co.,Ltd. 1972 SANKO METAL INDUSTRIAL CO.,LTD. 2196 ESCRIT INC. 2117 Nissin Sugar Co.,Ltd. 2198 IKK Inc. 2124 JAC Recruitment Co.,Ltd. 2418 TSUKADA GLOBAL HOLDINGS Inc. 2170 Link and Motivation Inc. 3079 DVx Inc. 2337 Ichigo Inc. 3093 Treasure Factory Co.,LTD. 2359 CORE CORPORATION 3194 KIRINDO HOLDINGS CO.,LTD. 2429 WORLD HOLDINGS CO.,LTD. 3205 DAIDOH LIMITED 2462 J-COM Holdings Co.,Ltd. 3667 enish,inc. 2485 TEAR Corporation 3834 ASAHI Net,Inc. 2492 Infomart Corporation 3946 TOMOKU CO.,LTD. 2915 KENKO Mayonnaise Co.,Ltd. 4221 Okura Industrial Co.,Ltd. 3179 Syuppin Co.,Ltd. 4238 Miraial Co.,Ltd. 3193 Torikizoku co.,ltd. 4331 TAKE AND GIVE. NEEDS Co.,Ltd. 3196 HOTLAND Co.,Ltd. 4406 New Japan Chemical Co.,Ltd. 3199 Watahan & Co.,Ltd. 4538 Fuso Pharmaceutical Industries,Ltd. 3244 Samty Co.,Ltd. 4550 Nissui Pharmaceutical Co.,Ltd. 3250 A.D.Works Co.,Ltd. 4636 T&K TOKA CO.,LTD. 3543 KOMEDA Holdings Co.,Ltd. 4651 SANIX INCORPORATED 3636 Mitsubishi Research Institute,Inc. 4809 Paraca Inc. 3654 HITO-Communications,Inc. 5204 ISHIZUKA GLASS CO.,LTD. 3666 TECNOS JAPAN INCORPORATED 5998 Advanex Inc. 3678 MEDIA DO Co.,Ltd. 6203 Howa Machinery,Ltd. 3688 VOYAGE GROUP,INC. 6319 SNT CORPORATION 3694 OPTiM CORPORATION 6362 Ishii Iron Works Co.,Ltd. 3724 VeriServe Corporation 6373 DAIDO KOGYO CO.,LTD. 3765 GungHo Online Entertainment,Inc. -

Pemafibrate, a Novel Selective Peroxisome Proliferator-Activated
www.nature.com/scientificreports OPEN Pemafibrate, a novel selective peroxisome proliferator-activated receptor alpha modulator, Received: 25 July 2016 Accepted: 11 January 2017 improves the pathogenesis in Published: 14 February 2017 a rodent model of nonalcoholic steatohepatitis Yasushi Honda1, Takaomi Kessoku1, Yuji Ogawa1, Wataru Tomeno1, Kento Imajo1, Koji Fujita1, Masato Yoneda1, Toshiaki Takizawa2, Satoru Saito1, Yoji Nagashima3 & Atsushi Nakajima1 The efficacy of peroxisome proliferator-activated receptorα -agonists (e.g., fibrates) against nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH) in humans is not known. Pemafibrate is a novel selective peroxisome proliferator-activated receptorα modulator that can maximize the beneficial effects and minimize the adverse effects of fibrates used currently. In a phase-2 study, pemafibrate was shown to improve liver dysfunction in patients with dyslipidaemia. In the present study, we first investigated the effect of pemafibrate on rodent models of NASH. Pemafibrate efficacy was assessed in a diet-induced rodent model of NASH compared with fenofibrate. Pemafibrate and fenofibrate improved obesity, dyslipidaemia, liver dysfunction, and the pathological condition of NASH. Pemafibrate improved insulin resistance and increased energy expenditure significantly. To investigate the effects of pemafibrate, we analysed the gene expressions and protein levels involved in lipid metabolism. We also analysed uncoupling protein 3 (UCP3) expression. Pemafibrate stimulated lipid turnover and upregulated UCP3 expression in the liver. Levels of acyl-CoA oxidase 1 and UCP3 protein were increased by pemafibrate significantly. Pemafibrate can improve the pathogenesis of NASH by modulation of lipid turnover and energy metabolism in the liver. Pemafibrate is a promising therapeutic agent for NAFLD/NASH. The incidence of nonalcoholic fatty liver disease (NAFLD) is increasing worldwide. -

A61p1/16 (2006.01) A61p3/00 (2006.01) Km, Ml, Mr, Ne, Sn, Td, Tg)
( (51) International Patent Classification: TR), OAPI (BF, BJ, CF, CG, Cl, CM, GA, GN, GQ, GW, A61P1/16 (2006.01) A61P3/00 (2006.01) KM, ML, MR, NE, SN, TD, TG). A61K 31/192 (2006.01) C07C 321/28 (2006.01) Declarations under Rule 4.17: (21) International Application Number: — as to the applicant's entitlement to claim the priority of the PCT/IB2020/000808 earlier application (Rule 4.17(iii)) (22) International Filing Date: Published: 25 September 2020 (25.09.2020) — with international search report (Art. 21(3)) (25) Filing Language: English — before the expiration of the time limit for amending the claims and to be republished in the event of receipt of (26) Publication Language: English amendments (Rule 48.2(h)) (30) Priority Data: 62/906,288 26 September 2019 (26.09.2019) US (71) Applicant: ABIONYX PHARMA SA [FR/FR] ; 33-43 Av¬ enue Georges Pompidou, Batiment D, 31130 Bahna (FR). (72) Inventor: DASSEUX, Jean-Louis, Henri; 7 Allees Charles Malpel, Bat. B, 31300 Toulouse (FR). (74) Agent: HOFFMANN EITLE PATENT- UND RECHTSANWALTE PARTMBB, ASSOCIATION NO. 151; Arabellastrasse 30, 81925 Munich (DE). (81) Designated States (unless otherwise indicated, for every kind of national protection available) : AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, IT, JO, JP, KE, KG, KH, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, WS, ZA, ZM, ZW. -

Effects of Pemafibrate on Glucose Metabolism Markers and Liver
Yokote et al. Cardiovasc Diabetol (2021) 20:96 https://doi.org/10.1186/s12933-021-01291-w Cardiovascular Diabetology ORIGINAL INVESTIGATION Open Access Efects of pemafbrate on glucose metabolism markers and liver function tests in patients with hypertriglyceridemia: a pooled analysis of six phase 2 and phase 3 randomized double‐blind placebo‐controlled clinical trials Koutaro Yokote1,2*, Shizuya Yamashita3, Hidenori Arai4, Eiichi Araki5, Mitsunori Matsushita6, Toshiaki Nojima7, Hideki Suganami7 and Shun Ishibashi8 Abstract Background: Increased risk of cardiovascular events is associated not only with dyslipidemias, but also with abnor- malities in glucose metabolism and liver function. This study uses pooled analysis to explore the in-depth efects of pemafbrate, a selective peroxisome proliferator-activated receptor α modulator (SPPARMα) already known to decrease elevated triglycerides, on glucose metabolism and liver function in patients with hypertriglyceridemia. Methods: We performed a post-hoc analysis of six phase 2 and phase 3 Japanese randomized double-blind placebo- controlled trials that examined the efects of daily pemafbrate 0.1 mg, 0.2 mg, and 0.4 mg on glucose metabolism markers and liver function tests (LFTs). Primary endpoints were changes in glucose metabolism markers and LFTs from baseline after 12 weeks of pemafbrate treatment. All adverse events and adverse drug reactions were recorded as safety endpoints. Results: The study population was 1253 patients randomized to placebo (n 298) or pemafbrate 0.1 mg/day (n 127), 0.2 mg/day (n 584), or 0.4 mg/day (n 244). Participant mean age= was 54.3 years, 65.4 % had BMI 25 kg/ m2=, 35.8 % had type 2 diabetes,= and 42.6 % had fatty= liver. -

1 Atb 2016-III Consolidated Business of Fiber & Textile Makers in Japan, April 1, 2019 to March 31, 2020
Consolidated Business of Fiber & Textile Makers in Japan, April 1, 2019 to March 31, 2020 (million yen) Y-o-Y Operating Y-o-Y Y-o-Y Net Sales Net Profits Change (%) Profits Change (%) Change (%) Fiber producers Toray 2,214,633 –7.3 131,186 –7.3 55,725 –29.8 Asahi Kasei 2,151,646 –0.9 177,264 –15.4 103,931 –29.5 Teijin 853,746 –3.9 56,205 –6.3 25,252 –44.0 Kaneka 601,514 –3.1 26,014 –27.8 14,003 –37.0 Toyobo 339,607 0.9 22,794 4.9 13,774 — Unitika 119,537 –7.4 5,467 –32.9 –2,158 — Kuraray(1) 575,807 –4.5 54,173 –17.7 –1,956 — Cotton spinners & textile makers Daiwabo Holdings 944,053 20.2 32,841 44.6 21,178 26.2 Kurabo 142,926 –9.0 4,541 –19.5 3,731 –19.7 Shikibo 38,037 –6.8 1,979 –17.7 983 — Fujibo Holdings 38,701 4.3 4,079 7.9 2,269 –10.6 Omikenshi 9,026 –7.4 –207 — –2,367 — Nitto Boseki 85,722 4.2 8,160 –0.5 5,771 –27.7 Nisshinbo Holdings(2) 509,660 — 6,284 — –6,604 — Textile dyers & processors Seiren 120,258 –2.0 10,502 –0.8 8,551 3.9 Komatsu Matere 36,525 –6.5 1,612 –25.5 1,375 –35.5 Sakai Ovex 27,561 1.1 2,123 4.9 2,313 3.8 Tokai Senko 14,010 –3.4 617 –17.9 –551 — Sotoh 11,219 –0.1 193 –19.1 –97 — Soko Seiren 2,778 –17.7 –245 — –130 — Note: (1) Kuraray's business performance is for the period from January to December 2019. -

Istoxx® Mutb Japan Momentum 300 Index
ISTOXX® MUTB JAPAN MOMENTUM 300 INDEX Components1 Company Supersector Country Weight (%) Z HOLDINGS Technology Japan 0.69 M3 Health Care Japan 0.65 KOEI TECMO HOLDINGS Technology Japan 0.65 MENICON Health Care Japan 0.59 CAPCOM Technology Japan 0.58 FUJITEC Industrial Goods & Services Japan 0.56 Ibiden Co. Ltd. Industrial Goods & Services Japan 0.56 NIPPON PAINT HOLDINGS Chemicals Japan 0.56 RENESAS ELECTRONICS Technology Japan 0.55 JEOL Industrial Goods & Services Japan 0.55 INTERNET INTV.JAPAN Technology Japan 0.53 JSR Corp. Chemicals Japan 0.52 NET ONE SYSTEMS Technology Japan 0.51 Fujitsu Ltd. Technology Japan 0.51 Bank of Kyoto Ltd. Banks Japan 0.51 Hokuhoku Financial Group Inc. Banks Japan 0.51 FUJITSU GENERAL Personal & Household Goods Japan 0.50 Iyo Bank Ltd. Banks Japan 0.50 Kyushu Financial Group Banks Japan 0.50 77 Bank Ltd. Banks Japan 0.49 COCOKARA FINE INC. Retail Japan 0.49 TOSHIBA TEC Industrial Goods & Services Japan 0.48 JCR PHARMACEUTICALS Health Care Japan 0.48 MONOTARO Retail Japan 0.48 COSMOS PHARM. Retail Japan 0.48 Tokyo Electron Ltd. Technology Japan 0.48 Nomura Research Institute Ltd. Technology Japan 0.48 Olympus Corp. Health Care Japan 0.47 SUNDRUG Retail Japan 0.47 Chiba Bank Ltd. Banks Japan 0.47 NEC NETWORKS & SY.INTG. Technology Japan 0.47 Nomura Holdings Inc. Financial Services Japan 0.47 TOKYO OHKA KOGYO Technology Japan 0.47 PENTA-OCEAN CONSTRUCTION Construction & Materials Japan 0.47 FUYO GENERAL LEASE Financial Services Japan 0.46 FUJI Industrial Goods & Services Japan 0.46 Hachijuni Bank Ltd. -

Stoxx® Japan 600 Esg-X Index
STOXX® JAPAN 600 ESG-X INDEX Components1 Company Supersector Country Weight (%) Toyota Motor Corp. Automobiles & Parts Japan 3.87 Sony Corp. Consumer Products & Services Japan 2.55 Softbank Group Corp. Telecommunications Japan 2.44 Keyence Corp. Industrial Goods & Services Japan 1.77 RECRUIT HOLDINGS Industrial Goods & Services Japan 1.54 Mitsubishi UFJ Financial Group Banks Japan 1.48 Shin-Etsu Chemical Co. Ltd. Chemicals Japan 1.36 Nippon Telegraph & Telephone C Telecommunications Japan 1.36 Nintendo Co. Ltd. Consumer Products & Services Japan 1.30 Nidec Corp. Technology Japan 1.30 Fast Retailing Co. Ltd. Retail Japan 1.25 Daikin Industries Ltd. Construction & Materials Japan 1.19 Takeda Pharmaceutical Co. Ltd. Health Care Japan 1.18 Tokyo Electron Ltd. Technology Japan 1.16 Honda Motor Co. Ltd. Automobiles & Parts Japan 1.10 Daiichi Sankyo Co. Ltd. Health Care Japan 1.08 Sumitomo Mitsui Financial Grou Banks Japan 1.04 Murata Manufacturing Co. Ltd. Technology Japan 1.03 KDDI Corp. Telecommunications Japan 1.02 Hitachi Ltd. Industrial Goods & Services Japan 0.92 Itochu Corp. Industrial Goods & Services Japan 0.92 Fanuc Ltd. Industrial Goods & Services Japan 0.90 Hoya Corp. Health Care Japan 0.84 Mitsubishi Corp. Industrial Goods & Services Japan 0.83 Mizuho Financial Group Inc. Banks Japan 0.76 SOFTBANK Telecommunications Japan 0.75 Denso Corp. Automobiles & Parts Japan 0.72 Mitsui & Co. Ltd. Industrial Goods & Services Japan 0.71 Tokio Marine Holdings Inc. Insurance Japan 0.70 Oriental Land Co. Ltd. Travel & Leisure Japan 0.68 SMC Corp. Industrial Goods & Services Japan 0.68 Mitsubishi Electric Corp. Industrial Goods & Services Japan 0.67 Seven & I Holdings Co. -

Accelerate Your Pathway to Partnerships in Asia MARCH 10-11, 2020 • TOKYO, JAPAN
Asia Tokyo International Conference 2020 Accelerate your Pathway to Partnerships in Asia MARCH 10-11, 2020 • TOKYO, JAPAN General Overview • Advisory Committee • Education and Conference Programming • Partnering • Sponsorship Opportunities • #BIOASIA20 • bio.org/asia The BIO Asia International Conference is an exclusive partnering forum that brings together the global biotechnology and pharmaceutical industry to explore licensing and investor engagement in the current Asia-Pacific business and policy environments. Event Attributes and Opportunities • Gain insights into the changes, challenges, and opportunities collaborations and funding opportunities with participating key opinion and policy leaders foresee for the Japanese companies; communicate directly with conference attendees; biotech market. and pre-schedule private, 30 minute One-on-One meetings to be conducted onsite. • Facilitate connections with strategic partners looking to invest, partner, or license in or license out, early to late-stage • Panel discussions will increase your understanding of, programs in your therapeutic area of focus. and interaction with, the Japanese biotech market, the political landscape in Japan, and its impact on this important Opportunity for organizations to deliver company • industry sector. presentations, providing increased visibility in front of a global audience of biotech and pharmaceutical companies, all • Network with government leaders, peers, investors, and interested in cross-border business development alliances and potential partners attending the conference and our exclusive research collaborations. welcome reception. • BIO One-on-One Partnering enables attendees to search • Exclusive sponsorship opportunities to showcase thought company and investor profiles, drug assets, products, leadership, elevate brand identity and maximize partnering and services in the biopharma industry; evaluate potential benefits with an elite audience. -

Istoxx® Mutb Japan Momentum 300 Index
ISTOXX® MUTB JAPAN MOMENTUM 300 INDEX Components1 Company Supersector Country Weight (%) SHIMACHU Retail Japan 0.83 LASERTEC Technology Japan 0.66 KADOKAWA Media Japan 0.65 KOEI TECMO HOLDINGS Technology Japan 0.64 M3 Health Care Japan 0.64 Taiyo Yuden Co. Ltd. Technology Japan 0.64 BAYCURRENT CONSULTING Technology Japan 0.63 AZBIL CORP. Industrial Goods & Services Japan 0.59 TOKYO OHKA KOGYO Technology Japan 0.58 DAIWABO HOLDINGS Industrial Goods & Services Japan 0.58 Ibiden Co. Ltd. Technology Japan 0.57 JEOL Industrial Goods & Services Japan 0.56 IR JAPAN HOLDINGS Industrial Goods & Services Japan 0.56 FUJI Industrial Goods & Services Japan 0.55 Tokyo Electron Ltd. Technology Japan 0.55 NIHON M&A CENTER Financial Services Japan 0.55 SUSHIRO GLOBAL HDG. Travel & Leisure Japan 0.55 Tokyo Century Corp Financial Services Japan 0.55 Advantest Corp. Technology Japan 0.54 CAPCOM Consumer Products & Services Japan 0.54 DAIFUKU Industrial Goods & Services Japan 0.54 NEXON Consumer Products & Services Japan 0.53 NIPPON GAS Utilities Japan 0.53 MONOTARO Retail Japan 0.52 TDK Corp. Technology Japan 0.50 TOKYO SEIMITSU Technology Japan 0.50 SHIFT Technology Japan 0.49 NIPPON PAINT HOLDINGS Industrial Goods & Services Japan 0.49 Sumco Corp. Technology Japan 0.48 Toho Gas Co. Ltd. Utilities Japan 0.48 KOBE BUSSAN Personal Care, Drug & Grocery Japan 0.48 Murata Manufacturing Co. Ltd. Technology Japan 0.47 Nomura Research Institute Ltd. Technology Japan 0.47 GMO PAYMENT GTWY. Industrial Goods & Services Japan 0.47 MIURA Industrial Goods & Services Japan 0.47 Obic Co. Ltd. -

NIKKEI JAPAN 1000 Constituents (As of March 25, 2005)
NIKKEI JAPAN 1000 Constituents (as of March 25, 2005) Tokyo Stock Exchange 1st 2580 COCA-COLA CENTRAL JAPAN 1332 NIPPON SUISAN 2590 DYDO DRINCO 1334 MARUHA GROUP 2591 CALPIS 1377 SAKATA SEED 2593 ITO EN 1379 HOKUTO 2594 KEY COFFEE 1601 TEIKOKU OIL 2595 KIRIN BEVERAGE 1662 JAPAN PETROLEUM EXPLORATION 2602 NISSHIN OILLIO GROUP 1720 TOKYU CONSTRUCTION 2607 FUJI OIL 1721 COMSYS HOLDINGS 2613 J-OIL MILLS 1722 MISAWA HOMES HOLDINGS 2651 LAWSON 1801 TAISEI 2664 CAWACHI 1802 OBAYASHI 2670 ABC-MART 1803 SHIMIZU 2678 ASKUL 1808 HASEKO 2681 GEO 1812 KAJIMA 2685 POINT 1820 NISHIMATSU CONSTRUCTION 2692 ITOCHU-SHOKUHIN 1821 SUMITOMO MITSUI CONSTRUCTION 2730 EDION 1824 MAEDA 2731 NIWS 1833 OKUMURA 2768 SOJITZ HOLDINGS 1860 TODA 2779 MITSUKOSHI 1878 DAITO TRUST CONSTRUCTION 2784 ALFRESA HOLDINGS 1881 NIPPO 2801 KIKKOMAN 1883 MAEDA ROAD CONSTRUCTION 2802 AJINOMOTO 1885 TOA 2809 Q.P. 1893 PENTA-OCEAN CONSTRUCTION 2810 HOUSE FOODS 1924 PANAHOME 2811 KAGOME 1925 DAIWA HOUSE INDUSTRY 2815 ARIAKE JAPAN 1928 SEKISUI HOUSE 2871 NICHIREI 1941 CHUDENKO 2873 KATOKICHI 1942 KANDENKO 2874 YOKOHAMA REITO 1946 TOENEC 2875 TOYO SUISAN 1951 KYOWA EXEO 2897 NISSIN FOOD PRODUCTS 1959 KYUDENKO 2908 FUJICCO 1961 SANKI ENGINEERING 2914 JAPAN TOBACCO 1963 JGC 3001 KATAKURA INDUSTRIES 1969 TAKASAGO THERMAL ENGINEERING 3002 GUNZE 1970 HITACHI PLANT ENGINEERING 3101 TOYOBO 1973 NEC SYS. INTEGRA. & CONST. 3103 UNITIKA 1979 TAIKISHA 3105 NISSHINBO INDUSTRIES 1982 HIBIYA ENGINEERING 3106 KURABO INDUSTRIES 2001 NIPPON FLOUR MILLS 3110 NITTO BOSEKI 2002 NISSHIN SEIFUN GROUP 3116 TOYOTA