A New Species of Colubrina (Rhamnaceae) of the Amazon Region of Ecuador
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Araracuara, Un Nuevo Género De Rhamnaceae De La Amazonía Colombiana
Volumen 65 N.º 2 julio-diciembre 2008 Madrid (España) ISSN: 0211-1322 CONSEJO SUPERIOR DE INVESTIGACIONES CIENTÍFICAS Anales del Jardín Botánico de Madrid Vol. 65(2): 343-352 julio-diciembre 2008 ISSN: 0211-1322 Araracuara, un nuevo género de Rhamnaceae de la Amazonía colombiana por José Luis Fernández-Alonso1 & María Victoria Arbeláez2 1 Instituto de Ciencias Naturales, Universidad Nacional de Colombia, Apartado Aéreo 7495, Bogotá D.C., Colombia. [email protected] 2 Institute for Biodiversity and Ecosystem Dynamics (IBED), Universiteit van Amsterdam, Kruislaan 318, 1098 SM, Amsterdam, The Netherlands. [email protected] Resumen Abstract Fernández-Alonso, J.L., & Arbeláez, M.V. 2008. Araracuara, un Fernández-Alonso, J.L., & Arbeláez, M.V. 2008. Araracuara, the nuevo género de Rhamnaceae de la Amazonía colombiana. Ana- new genera of the Rhamnaceae from Colombian Amazon. Ana- les Jard. Bot. Madrid 65(2): 343-352. les Jard. Bot. Madrid 65(2): 343-352 (in Spanish). Se describe e ilustra Araracuara Fern. Alonso, un nuevo género de Araracuara Fern. Alonso, a new genus of Rhamnaceae only la familia Rhamnaceae conocido tan sólo de las mesetas de arenisca known from the sandstone plateaus of the Colombian Amazon, de la Amazonía colombiana. Se discuten sus posibles afinidades en is described and illustrated. Its possible affinities are discussed la familia y se sugiere que estaríamos ante un género relíctico, rela- and it is proposed that this is a relictual genus related to the pan- cionado con el pantropical Colubrina y en menor medida con el tropical Colubrina and to a lesser degree with the Amazonian amazónico Ampelozizyphus. -

Forest Succession in Tropical Hardwood Hammocks of the Florida Keys: Effects of Direct Mortality from Hurricane Andrew1
BIOTROPICA 33(1): 23±33 2001 Forest Succession in Tropical Hardwood Hammocks of the Florida Keys: Effects of Direct Mortality from Hurricane Andrew1 Michael S. Ross2 Florida International University, Southeast Environmental Research Center, University Park, Miami, Florida 33199, U.S.A. Mary Carrington Environmental Biology, Governors State University, University Park, Illinois 60466, U.S.A. Laura J. Flynn The Nature Conservancy, Lower Hudson Chapter, 41 South Moger Avenue, Mt. Kisco, New York 10549, U.S.A. Pablo L. Ruiz Florida International University, Southeast Environmental Research Center, University Park, Miami, Florida 33199, U.S.A. ABSTRACT A tree species replacement sequence for dry broadleaved forests (tropical hardwood hammocks) in the upper Florida Keys was inferred from species abundances in stands abandoned from agriculture or other anthropogenic acitivities at different times in the past. Stands were sampled soon after Hurricane Andrew, with live and hurricane-killed trees recorded separately; thus it was also possible to assess the immediate effect of Hurricane Andrew on stand successional status. We used weighted averaging regression to calculate successional age optima and tolerances for all species, based on the species composition of the pre-hurricane stands. Then we used weighted averaging calibration to calculate and compare inferred successional ages for stands based on (1) the species composition of the pre-hurricane stands and (2) the hurricane-killed species assemblages. Species characteristic of the earliest stages of post-agricultural stand de- velopment remains a signi®cant component of the forest for many years, but are gradually replaced by taxa not present, even as seedlings, during the ®rst few decades. -

Colubrina Arborescens 1 Edward F
Fact Sheet FPS-137 October, 1999 Colubrina arborescens 1 Edward F. Gilman2 Introduction Description Native to south Florida in the coastal upland plant Height: 15 to 25 feet community and the Caribbean Basin, this small tree or large Spread: 12 to 20 feet shrub can reach a height of 20 feet or more (Fig. 1). Handsome, Plant habit: round; oval shiny leaves are borne on thin twigs covered with rust-colored Plant density: dense hairs. Hairs occasionally extend onto the underside of leaves. Growth rate: slow Prominent yellow veins contrast with the dark green leaves. Texture: medium Plants grow in dense clusters in sunny or partially shaded locations. Foliage General Information Leaf arrangement: alternate Leaf type: simple Leaf margin: entire Scientific name: Colubrina arborescens Leaf shape: ovate Pronunciation: kawl-yoo-BRYE-nuh ar-bor-RESS-enz Leaf venation: pinnate Common name(s): Coffee Colubrina, Wild Coffee Leaf type and persistence: evergreen Family: Rhamnaceae Leaf blade length: 2 to 4 inches Plant type: tree Leaf color: green USDA hardiness zones: 10B through 11 (Fig. 1) Fall color: no fall color change Planting month for zone 10 and 11: year round Fall characteristic: not showy Origin: native to Florida Uses: container or above-ground planter; reclamation plant; Flower trained as a standard; hedge; near a deck or patio; specimen; espalier; small parking lot islands (< 100 square feet in size); Flower color: yellow medium-sized parking lot islands (100-200 square feet in size); Flower characteristic: year-round flowering large -

Colubrina Asiatica
Colubrina asiatica Colubrina asiatica Asiatic colubrine, latherleaf Introduction The genus Colubrina contains approximately 23 species, distributed in South Asia, islands of Oceania, Africa, and Latin America. Two species reportedly occur in Guangdong, Guangxi, Taiwan, and Yunnan provinces of China[11]. Species of Colubrina in China C. asiatica (L.) Brongn C. pubescens Kurz. Leaves of Colubrina asiatica. (Photo pro- glabrous or nearly so, ovate or broadly vided by TNC.) ovate in shape, 4-8 cm long and 2-5 cm Taxonomy wide, with two or three raised lateral rounded, hooded, and equal in length to Family: Rhamnaceae veins. The leaf margin is crenate, the stamen. Fruits appear from September Genus: Colubrina Rich. ex apex acuminate and slightly notched, to December as capsule-like drupes, Brongn. and base round or subcordate. Cyme globose, and 7-9 mm in diameter. A inflorescences of yellow flowers appear single grayish- brown seed is enclosed Description in the axils from June to September. in each of three pyrenes[11]. Colubrina asiatica is a vine-like The calyx is five-lobed; each sepal is glabrescent shrub. Alternate leaves are ovately-triangular. Petals are obovate- Habitat and Distribution nearly membranous or thinly papery, Colubrina asiatica occurs in forested areas and brush along the coast. Arthropods Economic Importance Order Family Species H. R. Ref. No information is available on the Artimpaza argenteonotata Pic m 16 economic importance of C. asiatica Coleoptera Cerambycidae in China. Niphona parallela White po 16 Paracopta Related Species Hemiptera Plataspidae p 192 duodecimpunctatum (Germar) Colubrina pubescens is distinguished from Asiatic colubrine in that the young shoots and veins on the underside of the leaves of C. -

Taxonomic Notes on Colubrina (Rhamnaceae)
Nesom, G.L. 2013. Taxonomic notes on Colubrina (Rhamnaceae). Phytoneuron 2013-4: 1–21. Published 28 January 2013. ISSN 2153 733X TAXONOMIC NOTES ON COLUBRINA (RHAMNACEAE) GUY L. NESOM 2925 Hartwood Drive Fort Worth, Texas 76109 [email protected] ABSTRACT Colubrina stricta Engelm. ex Blankinship (not Engelm. ex M.C. Johnston) has previously been regarded as a rare and widely scattered species of Texas and northern Coahuila and Nuevo León, Mexico, but is treated here as including C. texensis var. pedunculata , a taxon of Chihuahua, Coahuila, and Durango, much broadening its range. Colubrina texensis (Torrey & A. Gray) A. Gray occurs in south-central Texas and in northern Coahuila, Nuevo León, and Tamaulipas and is partly sympatric with C. stricta but morphologically distinct and apparently non-intergrading. The isolated population system identified as C. stricta in El Paso County is geographically disjunct and atypical in morphology but variants occur elsewhere in the overall range as well. Colubrina greggii S. Wats. has previously been regarded as having three varieties, but each of the three is treated here at specific rank: Colubrina greggii sensu stricto is widespread in eastern Mexico, with a single population in southeastern Texas; Colubrina angustior (M.C. Johnston) Nesom, comb. et stat. nov., apparently is relatively narrowly localized in east-central Mexico (San Luis Potosí, Veracruz, and Tamaulipas); Colubrina yucatanensis (M.C. Johnston) Nesom, comb. et stat. nov., is an abundant species of the Yucatán Peninsula (Petén, Guatemala, and Campeche, Quintana Roo, and Yucatán), long-disjunct from the range of typical C. greggii . Geographic range maps and images showing variability are included. -
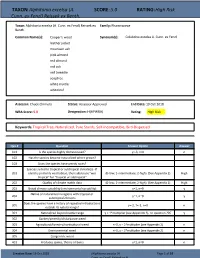
Alphitonia Excelsa (A
TAXON: Alphitonia excelsa (A. SCORE: 5.0 RATING: High Risk Cunn. ex Fenzl) Reissek ex Benth. Taxon: Alphitonia excelsa (A. Cunn. ex Fenzl) Reissek ex Family: Rhamnaceae Benth. Common Name(s): Cooper's wood Synonym(s): Colubrina excelsa A. Cunn. ex Fenzl leather jacket mountain ash pink almond red almond red ash red tweedie soaptree white myrtle whiteleaf Assessor: Chuck Chimera Status: Assessor Approved End Date: 19 Oct 2018 WRA Score: 5.0 Designation: H(HPWRA) Rating: High Risk Keywords: Tropical Tree, Naturalized, Pure Stands, Self-Incompatible, Bird-Dispersed Qsn # Question Answer Option Answer 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? 103 Does the species have weedy races? Species suited to tropical or subtropical climate(s) - If 201 island is primarily wet habitat, then substitute "wet (0-low; 1-intermediate; 2-high) (See Appendix 2) High tropical" for "tropical or subtropical" 202 Quality of climate match data (0-low; 1-intermediate; 2-high) (See Appendix 2) High 203 Broad climate suitability (environmental versatility) y=1, n=0 y Native or naturalized in regions with tropical or 204 y=1, n=0 y subtropical climates Does the species have a history of repeated introductions 205 y=-2, ?=-1, n=0 n outside its natural range? 301 Naturalized beyond native range y = 1*multiplier (see Appendix 2), n= question 205 y 302 Garden/amenity/disturbance weed 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see Appendix 2) n 304 Environmental weed n=0, y = 2*multiplier (see Appendix 2) n 305 Congeneric weed 401 Produces spines, thorns or burrs y=1, n=0 n Creation Date: 19 Oct 2018 (Alphitonia excelsa (A. -

Tribe Species Secretory Structure Compounds Organ References Incerteae Sedis Alphitonia Sp. Epidermis, Idioblasts, Cavities
Table S1. List of secretory structures found in Rhamanaceae (excluding the nectaries), showing the compounds and organ of occurrence. Data extracted from the literature and from the present study (species in bold). * The mucilaginous ducts, when present in the leaves, always occur in the collenchyma of the veins, except in Maesopsis, where they also occur in the phloem. Tribe Species Secretory structure Compounds Organ References Epidermis, idioblasts, Alphitonia sp. Mucilage Leaf (blade, petiole) 12, 13 cavities, ducts Epidermis, ducts, Alphitonia excelsa Mucilage, terpenes Flower, leaf (blade) 10, 24 osmophores Glandular leaf-teeth, Flower, leaf (blade, Ceanothus sp. Epidermis, hypodermis, Mucilage, tannins 12, 13, 46, 73 petiole) idioblasts, colleters Ceanothus americanus Idioblasts Mucilage Leaf (blade, petiole), stem 74 Ceanothus buxifolius Epidermis, idioblasts Mucilage, tannins Leaf (blade) 10 Ceanothus caeruleus Idioblasts Tannins Leaf (blade) 10 Incerteae sedis Ceanothus cordulatus Epidermis, idioblasts Mucilage, tannins Leaf (blade) 10 Ceanothus crassifolius Epidermis; hypodermis Mucilage, tannins Leaf (blade) 10, 12 Ceanothus cuneatus Epidermis Mucilage Leaf (blade) 10 Glandular leaf-teeth Ceanothus dentatus Lipids, flavonoids Leaf (blade) (trichomes) 60 Glandular leaf-teeth Ceanothus foliosus Lipids, flavonoids Leaf (blade) (trichomes) 60 Glandular leaf-teeth Ceanothus hearstiorum Lipids, flavonoids Leaf (blade) (trichomes) 60 Ceanothus herbaceus Idioblasts Mucilage Leaf (blade, petiole), stem 74 Glandular leaf-teeth Ceanothus -

Nomenclatural Notes on the Alphitonia Group in Australia (Rhamnaceae) Jürgen Kellermanna,B
Swainsona 33: 135–142 (2020) © 2020 Board of the Botanic Gardens & State Herbarium (Adelaide, South Australia) Nomenclatural notes on the Alphitonia Group in Australia (Rhamnaceae) Jürgen Kellermanna,b a State Herbarium of South Australia, Botanic Gardens and State Herbarium, Hackney Road, Adelaide, South Australia 5000 Email: [email protected] b The University of Adelaide, School of Biological Sciences, Adelaide, South Australia 5005 Abstract: The nomenclature and typification of seven species of Alphitonia Reissek ex Endl. and Emmenosperma F.Muell. is discussed. These two genera form the “Alphitonia Group” together with Granitites Rye and Jaffrea H.C.Hopkins & Pillon from New Caledonia. Lectotypes are chosen for E. cunninghamii Benth. and for the synonyms A. excelsa var. acutifolia Braid, A. obtusifolia R.Br. ex Braid and A. obtusifolia var. tenuis Braid. The lectotypes of A. petriei Braid & C.T.White and A. philippinensis Braid are clarified. Three species are illustrated: A. petriei, A. whitei Braid and E. cunninghamii. Keywords: Nomenclature, typification, second-step lectotype, Rhamnaceae, incertae sedis, Australia Introduction when fruits are ripe, they split open and the pericarp falls off, leaving the seeds (usually 3) remaining attached Rhamnaceae Juss. is a medium-sized plant family with to the base of the fruit (Fig. 3F, P). over 1000 species in 64 genera. The current intra- familial classification was devised by Richardson et al. Alphitonia (10–15 species) was published in (2000b), following the first molecular analysis of the Endlicher (1840), using Reissek’s manuscript name whole family (Richardson et al. 2000a). The family is and description. He indicated that Colubrina excelsa divided into 11 tribes, but several genera have not been A.Cunn. -

On the Flora of Australia
L'IBRARY'OF THE GRAY HERBARIUM HARVARD UNIVERSITY. BOUGHT. THE FLORA OF AUSTRALIA, ITS ORIGIN, AFFINITIES, AND DISTRIBUTION; BEING AN TO THE FLORA OF TASMANIA. BY JOSEPH DALTON HOOKER, M.D., F.R.S., L.S., & G.S.; LATE BOTANIST TO THE ANTARCTIC EXPEDITION. LONDON : LOVELL REEVE, HENRIETTA STREET, COVENT GARDEN. r^/f'ORElGN&ENGLISH' <^ . 1859. i^\BOOKSELLERS^.- PR 2G 1.912 Gray Herbarium Harvard University ON THE FLORA OF AUSTRALIA ITS ORIGIN, AFFINITIES, AND DISTRIBUTION. I I / ON THE FLORA OF AUSTRALIA, ITS ORIGIN, AFFINITIES, AND DISTRIBUTION; BEIKG AN TO THE FLORA OF TASMANIA. BY JOSEPH DALTON HOOKER, M.D., F.R.S., L.S., & G.S.; LATE BOTANIST TO THE ANTARCTIC EXPEDITION. Reprinted from the JJotany of the Antarctic Expedition, Part III., Flora of Tasmania, Vol. I. LONDON : LOVELL REEVE, HENRIETTA STREET, COVENT GARDEN. 1859. PRINTED BY JOHN EDWARD TAYLOR, LITTLE QUEEN STREET, LINCOLN'S INN FIELDS. CONTENTS OF THE INTRODUCTORY ESSAY. § i. Preliminary Remarks. PAGE Sources of Information, published and unpublished, materials, collections, etc i Object of arranging them to discuss the Origin, Peculiarities, and Distribution of the Vegetation of Australia, and to regard them in relation to the views of Darwin and others, on the Creation of Species .... iii^ § 2. On the General Phenomena of Variation in the Vegetable Kingdom. All plants more or less variable ; rate, extent, and nature of variability ; differences of amount and degree in different natural groups of plants v Parallelism of features of variability in different groups of individuals (varieties, species, genera, etc.), and in wild and cultivated plants vii Variation a centrifugal force ; the tendency in the progeny of varieties being to depart further from their original types, not to revert to them viii Effects of cross-impregnation and hybridization ultimately favourable to permanence of specific character x Darwin's Theory of Natural Selection ; — its effects on variable organisms under varying conditions is to give a temporary stability to races, species, genera, etc xi § 3. -

Lepidoptera: Pyraloidea: Crambidae) Inferred from DNA and Morphology 141-204 77 (1): 141 – 204 2019
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Arthropod Systematics and Phylogeny Jahr/Year: 2019 Band/Volume: 77 Autor(en)/Author(s): Mally Richard, Hayden James E., Neinhuis Christoph, Jordal Bjarte H., Nuss Matthias Artikel/Article: The phylogenetic systematics of Spilomelinae and Pyraustinae (Lepidoptera: Pyraloidea: Crambidae) inferred from DNA and morphology 141-204 77 (1): 141 – 204 2019 © Senckenberg Gesellschaft für Naturforschung, 2019. The phylogenetic systematics of Spilomelinae and Pyraustinae (Lepidoptera: Pyraloidea: Crambidae) inferred from DNA and morphology Richard Mally *, 1, James E. Hayden 2, Christoph Neinhuis 3, Bjarte H. Jordal 1 & Matthias Nuss 4 1 University Museum of Bergen, Natural History Collections, Realfagbygget, Allégaten 41, 5007 Bergen, Norway; Richard Mally [richard. [email protected], [email protected]], Bjarte H. Jordal [[email protected]] — 2 Florida Department of Agriculture and Consumer Ser- vices, Division of Plant Industry, 1911 SW 34th Street, Gainesville, FL 32608 USA; James E. Hayden [[email protected]] — 3 Technische Universität Dresden, Institut für Botanik, 01062 Dresden, Germany; Christoph Neinhuis [[email protected]] — 4 Senckenberg Naturhistorische Sammlungen Dresden, Museum für Tierkunde, Königsbrücker Landstraße 159, 01109 Dresden, Germany; Matthias Nuss [[email protected]] — * Corresponding author Accepted on March 14, 2019. Published online at www.senckenberg.de/arthropod-systematics on May 17, 2019. Published in print on June 03, 2019. Editors in charge: Brian Wiegmann & Klaus-Dieter Klass. Abstract. Spilomelinae and Pyraustinae form a species-rich monophylum of Crambidae (snout moths). Morphological distinction of the two groups has been diffcult in the past, and the morphologically heterogenous Spilomelinae has not been broadly accepted as a natural group due to the lack of convincing apomorphies. -

Colubrina Asiatica Click on Images to Enlarge
Species information Abo ut Reso urces Hom e A B C D E F G H I J K L M N O P Q R S T U V W X Y Z Colubrina asiatica Click on images to enlarge Family Rhamnaceae Scientific Name Colubrina asiatica (L.) Brongn. Brongniart, A.T. (1826) Memoire sur la Famille des Rhamnees : 62. Common name Leaves, flowers and immature fruit. Copyright CSIRO Colubrina; Beach Berry Bush Stem Usually grows as a vine but sometimes flowers and fruits as a shrub. Vine stem diameters to 6 cm recorded. Leaves Leaf blades about 3.5-10 x 2-7 cm, petioles about 1-1.5 cm long. Stipules small and inconspicuous, about 1- 1.5 mm long. Lateral veins 3 or 4 on each side of the midrib. Flowers Leaves and fruits. Copyright CSIRO Flowers about 4-5 mm diam. Calyx lobes triangular. Petals hooded, about 1 mm long, enclosing the stamens. Disk glandular, placed between the anther filaments and the style. Fruit Fruit depressed globular, about 7-9 x 8-10 mm, capsular but sitting in a saucer-shaped cupule about 6 mm diam. Seeds three per fruit, about 5-6 x 4-5 mm. Embryo about 4-5 x 4-5 mm. Seedlings Cotyledons about 13-18 x 11-15 mm, petioles hairy, about 2-3 mm long. First pair of leaves opposite, margins serrate, leaf blades 3-veined towards the base. Petioles about 5-6 mm long. Stipules about 1 mm Scale bar 10mm. Copyright CSIRO long. At the tenth leaf stage: leaf blade margin serrate, leaf blade 3-veined at the base. -

First Records of Butterflies (Lepidoptera)
First Records of Butterflies (Lepidoptera) from the Republic of Nauru1 Donald W. Buden2,3 and W. John Tennent4 Abstract: Four species of butterflies are reported from Nauru for the first time and as first records of butterflies from the island republic. None is endemic. Three of the four species are widespread in Oceania: Badamia exclamationis (Fabricius), Danaeus plexippus (Linnaeus), and Hypolimnas bolina (Linnaeus). The other, Petrelaea tombugensis (Ro¨ber), belongs to a genus that also is wide- spread in the Pacific. The small number of widespread species found on Nauru is comparable with the situation encountered on other small, remote, low-lying Pacific islands. In the most recent checklist of butterflies Study Area of Pacific Ocean islands, Tennent (2006) re- The Republic of Nauru (0 30 0 S, 166 56 0 E) marked on the absence of records from Na- consists of a single, small (21 km2), raised uru, Midway, and Johnston Atoll among the atoll island in the west-central Pacific Ocean, numerous states, countries, and isolated is- approximately 2,100 km northeast of New lands and island groups within this vast area. Guinea (Figure 1). The nearest island is Ba- Froggatt (1910:407) remarked that ‘‘butter- naba (¼ Ocean) Island, 300 km to the east. A flies were rare’’ on Nauru based on informa- roughly 100 to 300 m wide coastal belt abuts tion provided by F. W. Steel, who collected a scarp that rises about 30 to 40 m (maximum insects on Nauru on some earlier but un- specified date. Butterflies seen by Steel were elevation 72 m at Command Ridge) to form the edge of a central plateau.