5 Aroma Compounds
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Process Engineering for Bioflavour Production with Metabolically Active
Process engineering for bioflavour production with metabolically active yeasts - a mini-review Magnus Carlquist, Brian Gibson, Yonca Karagul Yuceer, Adamantini Paraskevopoulou, Mari Sandell, Angel I. Angelov, Velitchka Gotcheva, Angel D. Angelov, Marlene Etschmann, Gustavo de Billerbeck, et al. To cite this version: Magnus Carlquist, Brian Gibson, Yonca Karagul Yuceer, Adamantini Paraskevopoulou, Mari Sandell, et al.. Process engineering for bioflavour production with metabolically active yeasts - a mini-review. Yeast, Wiley, 2015, 32 (1), pp.123 - 143. 10.1002/yea.3058. hal-01269072 HAL Id: hal-01269072 https://hal.archives-ouvertes.fr/hal-01269072 Submitted on 28 May 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Yeast Yeast 2015; 32: 123–143. Published online 16 December 2014 in Wiley Online Library (wileyonlinelibrary.com) DOI: 10.1002/yea.3058 Special Issue Article Process engineering for bioflavour production with metabolically active yeasts – a mini-review Magnus Carlquist1, Brian Gibson2, Yonca Karagul Yuceer3, Adamantini Paraskevopoulou4, -

Medical Review Officer Manual
Department of Health and Human Services Substance Abuse and Mental Health Services Administration Center for Substance Abuse Prevention Medical Review Officer Manual for Federal Agency Workplace Drug Testing Programs EFFECTIVE OCTOBER 1, 2010 Note: This manual applies to Federal agency drug testing programs that come under Executive Order 12564 dated September 15, 1986, section 503 of Public Law 100-71, 5 U.S.C. section 7301 note dated July 11, 1987, and the Department of Health and Human Services Mandatory Guidelines for Federal Workplace Drug Testing Programs (73 FR 71858) dated November 25, 2008 (effective October 1, 2010). This manual does not apply to specimens submitted for testing under U.S. Department of Transportation (DOT) Procedures for Transportation Workplace Drug and Alcohol Testing Programs (49 CFR Part 40). The current version of this manual and other information including MRO Case Studies are available on the Drug Testing page under Medical Review Officer (MRO) Resources on the SAMHSA website: http://www.workplace.samhsa.gov Previous Versions of this Manual are Obsolete 3 Table of Contents Chapter 1. The Medical Review Officer (MRO)........................................................................... 6 Chapter 2. The Federal Drug Testing Custody and Control Form ................................................ 7 Chapter 3. Urine Drug Testing ...................................................................................................... 9 A. Federal Workplace Drug Testing Overview.................................................................. -

Color, Taste, and Odor: What You Should Know
Color, Taste, and Odor: What you should know From time to time the MassDEP receives consumer questions or complaints regarding the look, taste or the odor of drinking water. Listed below are common problems with drinking water and their most common causes. Please note that a particular problem in your drinking water may be the result of a cause not listed here; the only way to confirm a cause is to have a certified lab analyze the water and discuss the results with drinking water professional. If you receive water from a public drinking water system it is important to contact the Public Water Supply (PWS) before having a laboratory analyze the water. Information on private water testing is available. Filtering or treating the water may remedy persistent problems; however MassDEP does not recommend filtering or treating your water supply if your water is supplied by a MassDEP- approved PWS. MassDEP also does not regulate or recommend specific treatment systems for private home use. If you decide to use a filtration or treatment device in your home, the Department strongly encourages you to contact National Sanitation Foundation (NSF) for a list of approved devices. If you purchase a treatment device for private home use MassDEP also strongly recommends that it is maintained and provide active maintenance according to the manufacturer's instructions. Failure to maintain the equipment properly may make treatment ineffective and/or may create the potential for contamination. Common problems with drinking water are grouped into three categories: Color problems Taste / odor problems Particles in water If the problem with your water is not described here, if you are on a public water system please contact the public water department in your city or town or the MassDEP Drinking Water Program at your nearest regional MassDEP office. -

About the Authors Authors
1099 About the Authors Authors Artin Arshamian Chapter E.42 Karolinska Institutet Artin Arshamian earned his PhD at Stockholm University and is currently a postdoc- Department of Clinical Neuroscience toral fellow jointly at Karolinska Insitutet, Stockholm, and Radboud University, the Stockholm, Sweden Donders Institute for Brain, Cognition and Behaviour in Nijmegen, the Netherlands. Radboud University Centre for Language Studies, and Donders Institute for Brain, Cognition, and Behaviour Nijmegen, The Netherlands [email protected] Anat Arzi Chapter E.45 The Weizmann Institute of Science Anat Arzi is a postdoctoral fellow studying the interaction between olfaction and sleep Department of Neurobiology at the Department of Neurobiology at the Weizmann Institute of Science, Israel. She Rehovot, Israel completed her PhD and MSc in Neurobiology at the Weizmann Institute of Science [email protected] and her BSc at the Hebrew University of Jerusalem. Jinhe Bai Chapter B.9 U.S. Dept. of Agriculture – Agricultural Jinhe Bai received his PhD from Osaka Prefecture University. He worked Research Service at the Northwest A&F University Yangling, Osaka EPA, Oregon State US Horticultural Research Laboratory University, and the Produce Safety and Quality and Horticultural Research Fort Pierce, USA Laboratories in the US. Now at the US Horticultural Research Laboratory, [email protected] he works on flavor chemistry of fruits and vegetables, focusing on flavor and quality changes occurring during postharvest storage and processing. Nicolas Baldovini Chapter A.3 Université de Nice-Sophia Antipolis Nicolas Baldovini received his PhD from the University of Corsica. Institut de Chimie de Nice, UMR 7272 CNRS After postdoctoral work at the University Louis Pasteur in Strasbourg Nice, France and the University of Tokyo he was appointed Assistant Professor at the [email protected] Université de Nice-Sophia Antipolis. -
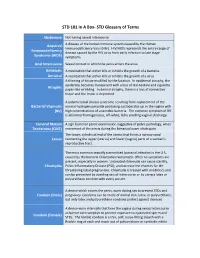
STD Glossary of Terms
STD 101 In A Box- STD Glossary of Terms Abstinence Not having sexual intercourse Acquired A disease of the human immune system caused by the Human Immunodeficiency Virus (HIV). HIV/AIDS represents the entire range of Immunodeficiency disease caused by the HIV virus from early infection to late stage Syndrome (AIDS) symptoms. Anal Intercourse Sexual contact in which the penis enters the anus. Antibiotic A medication that either kills or inhibits the growth of a bacteria. Antiviral A medication that either kills or inhibits the growth of a virus. A thinning of tissue modified by the location. In epidermal atrophy, the epidermis becomes transparent with a loss of skin texture and cigarette Atrophic paper-like wrinkling. In dermal atrophy, there is a loss of connective tissue and the lesion is depressed. A polymicrobial clinical syndrome resulting from replacement of the Bacterial Vaginosis normal hydrogen peroxide producing Lactobacillus sp. in the vagina with (BV) high concentrations of anaerobic bacteria. The common symptom of BV is abnormal homogeneous, off-white, fishy smelling vaginal discharge. Cervical Motion A sign found on pelvic examination suggestive of pelvic pathology; when Tenderness (CMT) movement of the cervix during the bimanual exam elicits pain. The lower, cylindrical end of the uterus that forms a narrow canal Cervix connecting the upper (uterus) and lower (vagina) parts of a woman's reproductive tract. The most common sexually transmitted bacterial infection in the U.S., caused by the bacteria Chlamydia trachomatis. Often no symptoms are present, especially in women. Untreated chlamydia can cause sterility, Chlamydia Pelvic Inflammatory Disease (PID), and increase the chances for life- threatening tubal pregnancies. -

A Pheromone Antagonist Liberates Female Sea Lamprey from a Sensory Trap to Enable Reliable Communication
A pheromone antagonist liberates female sea lamprey from a sensory trap to enable reliable communication Tyler J. Buchingera,1, Anne M. Scotta,1, Skye D. Fissettea, Cory O. Branta,2, Mar Huertasa,3,KeLia,4, Nicholas S. Johnsonb, and Weiming Lia,5 aDepartment of Fisheries and Wildlife, Michigan State University, East Lansing, MI 48824; and bHammond Bay Biological Station, US Geological Survey, Millersburg, MI 49759 Edited by John G. Hildebrand, University of Arizona, Tucson, AZ, and approved February 21, 2020 (received for review December 12, 2019) The evolution of male signals and female preferences remains a subsequent preference evolution precludes a full understanding central question in the study of animal communication. The sensory of how receiver biases shape communication. trap model suggests males evolve signals that mimic cues used Sea lamprey (Petromyzon marinus) are a useful model to track in nonsexual contexts and thus manipulate female behavior to the evolutionary trajectory of communication that originated via generate mating opportunities. Much evidence supports the sen- a sensory trap. Chemical cues and pheromones guide sea lam- sory trap model, but how females glean reliable information from prey behavior throughout their complex life history, especially both mimetic signals and their model cues remains unknown. We during the terminal reproductive phase (21). After parasitizing discovered a mechanism whereby a manipulative male signal guides fish in lakes or the Atlantic Ocean, prespawning sea lamprey reliable communication in sea lamprey (Petromyzon marinus). Mi- migrate into streams following chemical cues released by larvae gratory sea lamprey follow a larval cue into spawning streams; once residing in nursery habitats near spawning grounds. -

Etude De La Diversité Des Souches D'oenococcus Oeni Responsables
Etude de la diversit´edes souches d'Oenococcus oeni responsables de la fermentation malolactique des vins dans diff´erentes r´egionsvitivinicoles Mariette El Khoury To cite this version: Mariette El Khoury. Etude de la diversit´edes souches d'Oenococcus oeni responsables de la fermentation malolactique des vins dans diff´erentes r´egionsvitivinicoles. Sciences agricoles. Universit´ede Bordeaux, 2014. Fran¸cais. <NNT : 2014BORD0357>. <tel-01309805> HAL Id: tel-01309805 https://tel.archives-ouvertes.fr/tel-01309805 Submitted on 1 May 2016 HAL is a multi-disciplinary open access L'archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destin´eeau d´ep^otet `ala diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publi´esou non, lished or not. The documents may come from ´emanant des ´etablissements d'enseignement et de teaching and research institutions in France or recherche fran¸caisou ´etrangers,des laboratoires abroad, or from public or private research centers. publics ou priv´es. THÈSE PRÉSENTÉE POUR OBTENIR LE GRADE DE DOCTEUR DE L’UNIVERSITÉ DE BORDEAUX École doctorale des Sciences de la Vie et de la Santé Spécialité : Œnologie Par Mariette EL KHOURY Etude de la diversité des souches d’Oenococcus oeni responsables de la fermentation malolactique des vins dans différentes régions vitivinicoles Sous la direction de M. Patrick LUCAS Soutenue le 16 décembre 2014 Membres du jury : M. E. COTON Professeur à l’Université de Bretagne Occidentale Président M. A. BORDONS Professeur à l’Université Rovira i Virgili de Tarragone Rapporteur Mme. R. TOURDOT-MARECHAL Maître de Conférence à l’Université de Bourgogne Examinateur M. -

Taste and Odor Control, but Use As a Control Chemical Must Be Evaluated Carefully Due to the Formation of Thms and Chlorophenol When Organics Are Present
Taste and Odor Most customers judge the quality of drinking water by taste and odor. If the customer is satisfied with these qualities, it is assumed the water is safe to drink. Many harmful contaminants in water cannot be detected due to taste or smell and many of the contaminants found in drinking water that have a detectable taste or odor are not harmful. Sources of taste and odor problems can be found in surface water and groundwater. Source water protection involves the prevention of contaminants from entering the source. Surface water or groundwater may become contaminated by pollutants such as gasoline, industrial solvents or a wide variety of volatile organics. The removal of contaminants from surface water or groundwater is costly and may involve the use of aeration, powdered activated carbon, or both. If taste and odor must be controlled at the treatment plant, oxidation, aeration and adsorption can be effective in reducing taste and odor, and improved coagulation filtration. ODOR MEASUREMENTS One of the most common methods for measuring odor in water is the threshold odor test. It involves a series of flasks presented to an observer, who is told that some of the samples contain odors and that the series is arranged in order of increasing concentrations. The observer is also given a known odor-free blank for reference during the test. The observer compares the flasks in ascending order with the blank and then notes whether an odor is detected in any sample flask. Individuals vary in their reactions to certain types of odors. An odor stimulus that is agreeable to one may be disagreeable to another. -

Identification of the Most Aroma-Active Compounds in Strawberries: Variety Differences and the Effects of Heating on Strawberry Puree
IDENTIFICATION OF THE MOST AROMA-ACTIVE COMPOUNDS IN STRAWBERRIES: VARIETY DIFFERENCES AND THE EFFECTS OF HEATING ON STRAWBERRY PUREE By KURT F. SCHULBACH A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2002 This dissertation is dedicated to my parents, Helen and Herb Schulbach. ACKNOWLEDGMENTS I would like to express my sincere appreciation to the cochairmen of my committee, Dr. Charles Sims and Dr. Russell Rouseff. I would also like to thank the other members of my committee, Dr. Craig Chandler, Dr. Donald Huber, and Dr. Maurice Marshall. I give special thanks to the graduate students in Dr. Sims’ laboratory, especially Rena Schonbrun, for making the laboratory a great place to work. I would like to thank my parents for their many years of love, encouragement, and support. I would also like to express my sincere gratitude to my girlfriend, Diane Del Gobbo, for her love and support. The financial assistance I received from the University of Florida is greatly appreciated. iii TABLE OF CONTENTS page ACKNOWLEDGMENTS iii LIST OF TABLES vii LIST OF FIGURES ix ABSTRACT x CHAPTERS 1. INTRODUCTION 1 Genetic Studies to Improve Strawberry Flavor 2 Sensory Studies of Strawberry Flavor 3 Chemical Analysis of Strawberry Fruit 3 Gas Chromatography/Olfactometry 5 Strawberry Aroma 5 Objectives 6 2. LITERATURE REVIEW 8 Strawberry Aroma 8 Processing and Storage Effects 11 Chemical Analysis of Fruit Volatiles 13 Solvent Extraction 15 Dynamic Headspace 17 Solid-Phase Microextraction 21 Sensory Analysis of Fruit Volatiles 27 Discrimination Techniques 33 Descriptive Analysis 34 Integrating Chemical and Sensory Analysis 42 Gas Chromatography/Olfactometry 43 Aroma Recombination 54 IV V 3. -

A Study on Fragrance Finish on Modal Knit Fabric M
© 2021 JETIR March 2021, Volume 8, Issue 3 www.jetir.org (ISSN-2349-5162) A STUDY ON FRAGRANCE FINISH ON MODAL KNIT FABRIC M. Sharmila1 Dr.R.Divya2 PG Student Asst Professor Department of Costume Design and Fashion, PSG College of Arts and Science. ABSTRACT Innovative fragrance finish on Modal fabric New textile technologies have enabled the application of cosmetic ingredients on fabric to provide its functional benefit to the end-use product and therefore, cosmetic textiles are moving from research to the stage of commercialisation. Fragrance finish is one such finish that falls under this category. A fragrance is made from a pleasant smelling aroma compound. Aromachology is a science that studies the effects of fragrances on the human body and mind. It researches how scents can be used to induce relaxation and make life more pleasant. Finish was prepared and applied by two methods. Tests were conducted to check the performance properties of fragrance finish and the effect of finish on physical properties and mechanical properties of the fabric for unwashed samples. Comparative analysis of the two finishing application methods was done. Keywords: Modal fabric, knitted, fragrance finish, citronella oil, padding mangle. 1 INTRODUCTION Modal was first developed by Austria based Lenzing AG Company who trade marked the fabrics’ name, but now many manufacturers make their own versions.1 Single jerseys knitted structures are the primary material for intimate clothing as well for the sport wearing. The main expectation of such clothing is to provide higher level of comfort to the human being .2 Fabric Sourcing is on the selection of knitted fabric material of Modal is a wood pulp based cellulosic fiber, made out of pure wooden chips from the beech tree, technically as the European Schneider Zelkova tree. -

Cracking the Odor Code: Molecular and Cellular Deconstruction of the Olfactory Circuit of Drosophila Larvae Kenta Asahina
Rockefeller University Digital Commons @ RU Student Theses and Dissertations 2008 Cracking the Odor Code: Molecular and Cellular Deconstruction of the Olfactory Circuit of Drosophila Larvae Kenta Asahina Follow this and additional works at: http://digitalcommons.rockefeller.edu/ student_theses_and_dissertations Part of the Life Sciences Commons Recommended Citation Asahina, Kenta, "Cracking the Odor Code: Molecular and Cellular Deconstruction of the Olfactory Circuit of Drosophila Larvae" (2008). Student Theses and Dissertations. Paper 196. This Thesis is brought to you for free and open access by Digital Commons @ RU. It has been accepted for inclusion in Student Theses and Dissertations by an authorized administrator of Digital Commons @ RU. For more information, please contact [email protected]. Cracking the Odor Code: Molecular and Cellular Deconstruction of the Olfactory Circuit of Drosophila Larvae A Thesis Presented to the Faculty of The Rockefeller University in Partial Fulfillment of the Requirements for the degree of Doctor of Philosophy By Kenta Asahina June 2008 © Copyright by Kenta Asahina 2008 CRACKING THE ODOR CODE: MOLECULAR AND CELLULAR DECONSTRUCTION OF THE OLFACTORY CIRCUIT OF DROSOPHILA LARVAE Kenta Asahina, Ph.D. The Rockefeller University 2008 The Drosophila larva offers a powerful model system to investigate the general principles by which the olfactory system processes behaviorally relevant sensory stimuli. The numerically reduced larval olfactory system relieves the formidable molecular and cellular complexity found in other organisms. This thesis presents a study in four parts that investigates molecular and neuronal mechanisms of larval odor coding. First, the larval odorant receptor (OR) repertoire was characterized. ORs define the olfactory receptive range of an animal. -

Aroma Characteristics of Lavender Extract and Essential Oil from Lavandula Angustifolia Mill
molecules Article Aroma Characteristics of Lavender Extract and Essential Oil from Lavandula angustifolia Mill. Xiangyang Guo 1,* and Pu Wang 2,* 1 College of Chemistry and Environmental Engineering, Shenzhen University, Shenzhen 518060, China 2 Department of Agronomy, School of Life Sciences, Inner Mongolia University, Hohhot 010020, China * Correspondence: [email protected] (X.G.); [email protected] (P.W.); Tel.: +86-755-2655-7081 (X.G.); +86-471-499-2944 (P.W.) Received: 16 October 2020; Accepted: 20 November 2020; Published: 26 November 2020 Abstract: Lavender and its products have excellent flavor properties. However, most studies focus on the aroma profiles of lavender essential oil (LEO). The volatiles in lavender extracts (LEs), either in volatile compositions or their odor characteristics, have rarely been reported. In this study, the odor characteristics of LEs and LEO were comprehensively investigated by gas chromatography-mass spectrometry (GC-MS), coupled with sensory evaluation and principal chemical analysis (PCA). In addition, the extraction conditions of lavender extracts from inflorescences of Lavandula angustifolia Mill. were optimized. Under the optimal conditions of extraction, twice with 95% edible ethanol as the solvent, the LEs tended to contain the higher intensity of characteristic floral, herbal and clove-like odors as well as higher scores of overall assessment and higher amounts of linalool, linalool oxides I and II, linalyl acetate, lavandulyl acetate and total volatiles than LEO. PCA analysis showed that there were significant differences on the odor characteristics between LEO and LEs. The LEO, which was produced by steam distillation with a yield of 2.21%, had the lower intensity of floral, clove-like, medicine-like, pine-like and hay notes, a lower score of overall assessment and lower levels of linalool oxides I and II, linalyl acetate, lavandulyl acetate and total volatiles compared with LEs, whereas the relative contents of linalool and camphor in LEO were significantly higher than that in LEs.