1 Glass-Transition Phenomena in Polymer Blends Ioannis M
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Modified PPE-PS Americas
Noryl* resin Modifi ed PPE-PS Americas 2 SABIC Innovative Plastics 1. Introduction Noryl* resin is based on a modified PPO technology developed by SABIC Innovative Plastics. Noryl resin is an extremely versatile material - a miscible blend of PPO resin and polystyrene - and the basic properties may be modified to achieve a variety of characteristics. Contents 1. Introduction 4 2. Applications 6 3. Properties and design 10 3.1 General properties 10 3.2 Mechanical properties 10 3.3 Electrical properties 13 3.4 Flammability 13 3.5 Environmental resistance 14 3.6 Processability 14 3.7 Mold shrinkage 15 4. Processing 16 4.1 Pre-drying 16 4.2 Equipment 16 4.3 Processing conditions 17 4.4 Purging of the barrel 17 4.5 Recycling 17 5. Secondary operations 18 5.1 Welding 18 5.2 Adhesives 19 5.3 Mechanical assembly 20 5.4 Painting 20 5.5 Metalization 21 5.6 Laser marking 22 5.7 Foaming 22 SABIC Innovative Plastics 3 1. Introduction Noryl* modifi ed PPO resin PPE-PS Characteristics Typical characteristics include excellent dimensional stability, low mold shrinkage, low water absorption and very low creep behavior at elevated temperatures. These properties combined with an outstanding hydrolytic stability in hot and cold water, make Noryl resin an excellent potential candidate for fluid engineering, environmental and potable water applications. An outstanding feature of Noryl resin is its retention of tensile and flexural strength, even at elevated temperatures. The gradual reduction in modulus as temperature is increased, is a key advantage of this material. As a result, parts made or molded from Noryl resin may be used with predictable performance over a wide temperature range. -

Main-Chain Phosphorus-Containing Polymers for Therapeutic Applications
molecules Review Main-Chain Phosphorus-Containing Polymers for Therapeutic Applications Paul Strasser and Ian Teasdale * Institute of Polymer Chemistry, Johannes Kepler University Linz (JKU), Altenberger Straße 69, A-4040 Linz, Austria; [email protected] * Correspondence: [email protected] Received: 13 March 2020; Accepted: 4 April 2020; Published: 8 April 2020 Abstract: Polymers in which phosphorus is an integral part of the main chain, including polyphosphazenes and polyphosphoesters, have been widely investigated in recent years for their potential in a number of therapeutic applications. Phosphorus, as the central feature of these polymers, endears the chemical functionalization, and in some cases (bio)degradability, to facilitate their use in such therapeutic formulations. Recent advances in the synthetic polymer chemistry have allowed for controlled synthesis methods in order to prepare the complex macromolecular structures required, alongside the control and reproducibility desired for such medical applications. While the main polymer families described herein, polyphosphazenes and polyphosphoesters and their analogues, as well as phosphorus-based dendrimers, have hitherto predominantly been investigated in isolation from one another, this review aims to highlight and bring together some of this research. In doing so, the focus is placed on the essential, and often mutual, design features and structure–property relationships that allow the preparation of such functional materials. The first part of the review details the relevant features of phosphorus-containing polymers in respect to their use in therapeutic applications, while the second part highlights some recent and innovative applications, offering insights into the most state-of-the-art research on phosphorus-based polymers in a therapeutic context. -

Poly(Phenylene Ether) Based Amphiphilic Block Copolymers
polymers Review ReviewPoly(phenylene ether) Based Amphiphilic Poly(phenyleneBlock Copolymers ether) Based Amphiphilic Block Copolymers Edward N. Peters EdwardSABIC, Selkirk, N. Peters NY 12158, USA; [email protected]; Tel.: +1-518-475-5458 SABIC, Selkirk, NY 12158, USA; [email protected]; Tel.: +1-518-475-5458 Received: 14 August 2017; Accepted: 5 September 2017; Published: 8 September 2017 Received: 14 August 2017; Accepted: 5 September 2017; Published: 8 September 2017 Abstract: Polyphenylene ether (PPE) telechelic macromonomers are unique hydrophobic polyols Abstract: Polyphenylene ether (PPE) telechelic macromonomers are unique hydrophobic polyols which have been used to prepare amphiphilic block copolymers. Various polymer compositions which have been used to prepare amphiphilic block copolymers. Various polymer compositions have have been synthesized with hydrophilic blocks. Their macromolecular nature affords a range of been synthesized with hydrophilic blocks. Their macromolecular nature affords a range of structures structures including random, alternating, and di- and triblock copolymers. New macromolecular including random, alternating, and di- and triblock copolymers. New macromolecular architectures architectures can offer tailored property profiles for optimum performance. Besides reducing can offer tailored property profiles for optimum performance. Besides reducing moisture uptake and moisture uptake and making the polymer surface more hydrophobic, the PPE hydrophobic segment making the polymer surface more hydrophobic, the PPE hydrophobic segment has good compatibility has good compatibility with polystyrene (polystyrene-philic). In general, the PPE contributes to the with polystyrene (polystyrene-philic). In general, the PPE contributes to the toughness, strength, toughness, strength, and thermal performance. Hydrophilic segments go beyond their affinity for and thermal performance. Hydrophilic segments go beyond their affinity for water. -
Chapter 16-2
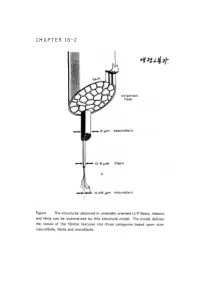
CHAPTER 16-2 ● Polymer Blends (reference) 1. Polymer-polymer Miscibility By Olabisi, Robeson, and Shaw Academic Press(1979) 2. Poplymer Blends, by D.R Paul, Ed, Academic Press (1978). 3. specific Interactions and the Miscibility of Polymer Blends, by Coleman, Graf, and Painter(1990) 4. Polymer Alloys and Blends, Thermodynamics and Rheology, by L.A. Utracki,(1990). ● Blend Preparation 방법: (a) Melt blending- screw extruder를 사용하여 blend하고자 하는 고분자를 용융점 이상에서 extrusion하여 mixing함. (b) Solution blending- mixing하고자 하는 고분자의 cosolvent를 사용하여 solution으로 만든 다음 cast하여 필름(film) 상태 로 blend를 제조함. (ex) polymer membrane(분리막)제조시. 그러나, amorphous polymer와 crystalline polymer를 blend했을때 는 film의 투명성으로부터 사용성을 판별하기는 어렵다. (2)DSC(differential scanning calorimetry): 시차 주사 열분석기를 이용하여 블렌드의 Tg(유리전이 온도)를 측 정함으로서 상용성을 관찰함-가장 많이 쓰이는 방법중 하나임. 즉 (i) a single phase exhibits one Tg. (ex) A polymer : Polycarbonate, Tg=150°C (423 K) B polymer : Polycaprolactane, Tg=-52°C (221 K) Blend (A+B)/50:50인 경우 w w 1 = A + B Tg Blend Tg A Tg B 1 = 0.5 + 0.5 (TgBlend=17.3°C (290.3 K) Tg Blend 423 221 (2) microscopy method (Scanning Electron microscopy). (3) FT-IR (Fourier Transform IR) (4) Ternary solution method (polymer 1- polymer 2 - solvent) - Compatible components from a single, transparent phase in mutual solution, while incompatible polymers exhibit phase seperation if the solution is not extremely dilute. - Equilibrium is relatively easily achived in dilutions and - Blends of immiscible (or partially miscible) materials can be useful so long as no significant desegration occurs while the mixture is being mixed. -

Dielectric Properties of Poly(Enaminonitrile)S
Polymer Journal, Vol. 32, No. 1, pp 57-61 (2000) Dielectric Properties of Poly(enaminonitrile)s Ji-Heung K1M,t Sang You! KIM,* James A. MOORE,** and James F. MASON*** Department ()f Chemical Engineering, Sungkyunkwan University, 300 Chunchun, Jangan, Suwon, Kyonggi 440-746, Korea * Department of Chemistry, Korea Advanced Institute of Science and Technology, 373-1 Kusung, Yusung, Taejon 305-701, Korea ** Department of Chemistry, Polymer Science and Engineering Program, Rensselaer Polytechnic Institute, Troy, NY 12180-3590, U.S.A. *** ATOCHEM N.A. King of Prussia, PA 19406--0018, U.S.A. (Received June 9, 1999) ABSTRACT: Poly(enaminonitrile)s (PEANs), a new class of thermally stable aromatic polymers, possess excellent thermal stability, good mechanical properties and are easily soluble in many organic solvents. Flexible, tough films can be cast from these solutions. These polymers undergo a 'curing' reaction at 300--350°C to insoluble, more dimensionally stable materials without evolution of volatile byproducts. We summarize here the dielectric data measured at a frequency of 100 kHz, 25°C for some PEANs, and discuss the results in relation to their structures. KEY WORDS Poly(enaminonitrile) / Dielectric Constant / Thermally Stable Polymer / Structure- Property / The production of thermally stable organic polymers reflects the alignment of dipoles and the net polariza has been the subject of many research efforts for the past tion of the molecules which is attributed to restricted 30 years because of the growing application of thermally movement of charges within the material. In an electric stable polymers in the fields of aerospace and elec field, positive charges move with the electric field and tronics.1 ·2 Most of thermally stable polymers synthesiz an equal number of negative charges move against it ed are aromatic, rigid-rod type polymers that have resulting in no net charge anywhere in the sample. -

Impact of the Pendant Group on the Chain Conformation and Bulk Properties of Norbornene Imide-Based Polymers † § ‡ § † ‡ Bret M
Article Cite This: Macromolecules XXXX, XXX, XXX−XXX pubs.acs.org/Macromolecules Impact of the Pendant Group on the Chain Conformation and Bulk Properties of Norbornene Imide-Based Polymers † § ‡ § † ‡ Bret M. Boyle, , Ozge Heinz, , Garret M. Miyake,*, and Yifu Ding*, † Department of Chemistry, Colorado State University, Fort Collins, Colorado 80523, United States ‡ Department of Mechanical Engineering, University of Colorado, Boulder, Colorado 80309, United States *S Supporting Information ABSTRACT: Three series of well-defined norbornene imide-based polymers with different pendant groups were synthesized to investigate the effect of the pendant group on the polymer conformation in solution and bulk melt properties. Each of these three series was examined by analyzing the polymers’ bulk z-average 8 (UTC). radius of gyration via static light scattering and the polymers’ melt viscoelastic properties via oscillatory measurements and differential scanning calorimetry. Sterically bulky pendant wedge groups modestly increase the rodlike conformation of the norbornene- imide polymer, however, the inherent rigidity of the polymer main- chain can still be observed with less bulky substituents. In stark contrast, the different side groups significantly impacted the bulk o legitimately share published articles. viscoelastic and thermal properties. By increasing the pendant group size, the chain diameter of the polymer increases and lowers the entanglement modulus. Finally, as the wedge pendant group size increases, the segmental relaxation time and the fragility index of these norbornene-based polymers are decreased. ■ INTRODUCTION groups extending away from the backbone that contain one or Bottlebrush polymers are molecules containing densely spaced more branch points in which another generation of branches begins. Often, these dendronized polymers can be directly polymeric side chains grafted onto a central backbone. -

Determination of Polymer Blend Composition, TS-22
Thermal Analysis & Rheology THERMAL SOLUTIONS DETERMINATION OF POLYMER BLEND COMPOSITION PROBLEM SOLUTION The blending of two or more polymers is becoming a Conventional DSC is a technique which measures the total common method for developing new materials for demand- heat flow into and out of a material as a function of ing applications such as impact-resistant parts and temperature and/or time. Hence, conventional DSC packaging films. Since the ultimate properties of blends can measures the sum of all thermal events occurring in the TM be significantly affected by what polymers are present, as material. Modulated DSC , on the other hand, is a new well as by small changes in the blend composition, technique which subjects a material to a linear heating suppliers of these materials are interested in rapid tests method which has a superimposed sinusoidal temperature which provide verification that the correct polymers and oscillation (modulation) resulting in a cyclic heating profile. amount of each polymer are present in the blend. Differen- tial scanning calorimetry (DSC) has proven to be an Deconvolution of the resultant heat flow profile during this effective technique for characterizing blends such as cyclic heating provides not only the "total" heat flow polyethylene/polypropylene where the crystalline melting obtained from conventional DSC, but also separates that endotherms or other transitions (e.g., glass transition) "total" heat flow into its heat capacity-related (reversing) associated with the polymer components are sufficiently and kinetic (nonreversing) components. It is this separa- TM separated to allow identification and/or quantitation. tion aspect which allows MDSC to more completely However, many blends do not exhibit this convenient evaluate polymer blend compositions. -

Spherical Polybutylene Terephthalate (PBT)—Polycarbonate (PC) Blend Particles by Mechanical Alloying and Thermal Rounding
polymers Article Spherical Polybutylene Terephthalate (PBT)—Polycarbonate (PC) Blend Particles by Mechanical Alloying and Thermal Rounding Maximilian A. Dechet 1,2,3,†, Juan S. Gómez Bonilla 1,2,3,†, Lydia Lanzl 3,4, Dietmar Drummer 3,4, Andreas Bück 1,2,3, Jochen Schmidt 1,2,3 and Wolfgang Peukert 1,2,3,* 1 Institute of Particle Technology, Friedrich-Alexander-Universität Erlangen-Nürnberg, Cauerstraße 4, D-91058 Erlangen, Germany; [email protected] (M.A.D.); [email protected] (J.S.G.B.); [email protected] (A.B.); [email protected] (J.S.) 2 Interdisciplinary Center for Functional Particle Systems, Friedrich-Alexander-Universität Erlangen-Nürnberg, Haberstraße 9a, D-91058 Erlangen, Germany 3 Collaborative Research Center 814—Additive Manufacturing, Am Weichselgarten 9, D-91058 Erlangen, Germany; [email protected] (L.L.); [email protected] (D.D.) 4 Institute of Polymer Technology, Friedrich-Alexander-Universität Erlangen-Nürnberg, Am Weichselgarten 9, D-91058 Erlangen, Germany * Correspondence: [email protected]; Tel.: +49-9131-85-29400 † The authors contributed equally. Received: 22 November 2018; Accepted: 7 December 2018; Published: 11 December 2018 Abstract: In this study, the feasibility of co-grinding and the subsequent thermal rounding to produce spherical polymer blend particles for selective laser sintering (SLS) is demonstrated for polybutylene terephthalate (PBT) and polycarbonate (PC). The polymers are jointly comminuted in a planetary ball mill, and the obtained product particles are rounded in a heated downer reactor. The size distribution of PBT–PC composite particles is characterized with laser diffraction particle sizing, while the shape and morphology are investigated via scanning electron microscopy (SEM). -

Advances in Polycarbodiimide Chemistry
Polymer 52 (2011) 1693e1710 Contents lists available at ScienceDirect Polymer journal homepage: www.elsevier.com/locate/polymer Feature Article Advances in polycarbodiimide chemistry Justin G. Kennemur, Bruce M. Novak* Department of Chemistry, North Carolina State University, USA article info abstract Article history: Over the last 15 years, large strides have been taken with regards to synthesis, catalysis, structural Received 21 December 2010 control, and functional application of polycarbodiimides (polyguanidines); a unique class of helical Received in revised form macromolecules that are derived from the polymerization of carbodiimide monomers using transition 13 February 2011 metal catalysis. This manuscript will provide a summary of the synthesis and characterization studies in Accepted 18 February 2011 addition to the large variety of properties discovered for these systems and potential applications Available online 3 March 2011 associated with these properties. In large part, it is the chiral helical backbone coupled with synthetically selective pendant groups which has allowed creation of polycarbodiimides spanning a large range of Keywords: Helical polymer potential applications. Such applications include cooperative chiral materials, liquid crystalline materials, Chiral polymer optical sensors and display materials, tunable polarizers, sites for asymmetric catalysis, and recyclable Polycarbodiimide polymers. Manipulation of the two pendant groups that span away from the amidine backbone repeat Polyguanidine unit has allowed solubility of these polymers in solvents ranging from cyclohexane to water, maximizing Asymmetric Catalysis the potential application of these polymers in various media. Liquid Crystals Ó 2011 Elsevier Ltd. All rights reserved. Chiro-optical Materials 1. Introduction further specificity will require their full description as non-linear or helical polycarbodiimides. -

Designing Polymer Blends Using Neural Networks, Genetic
Designing Polymer Blends Using Neural Networks, Genetic Algorithms, and Markov Chains N. K. Roy1,2, W. D. Potter1, D. P. Landau2 1Department of Computer Science University of Georgia, Athens, GA 30602 2Center for Simulational Physics University of Georgia, Athens, GA 30602 ABSTRACT In this paper we present a new technique to simulate polymer blends that overcomes the shortcomings in polymer system modeling. This method has an inherent advantage in that the vast existing information on polymer systems forms a critical part in the design process. The stages in the design begin with selecting potential candidates for blending using Neural Networks. Generally the parent polymers of the blend need to have certain properties and if the blend is miscible then it will reflect the properties of the parents. Once this step is finished the entire problem is encoded into a genetic algorithm using various models as fitness functions. We select the lattice fluid model of Sanchez and Lacombe1, which allows for a compressible lattice. After reaching a steady-state with the genetic algorithm we transform the now stochastic problem that satisfies detailed balance and the condition of ergodicity to a Markov Chain of states. This is done by first creating a transition matrix, and then using it on the incidence vector obtained from the final populations of the genetic algorithm. The resulting vector is converted back into a 1 population of individuals that can be searched to find the individuals with the best fitness values. A high degree of convergence not seen using the genetic algorithm alone is obtained. We check this method with known systems that are miscible and then use it to predict miscibility on several unknown systems. -

Phase Behavior of Polymer Solutions and Blends Piotr Knychała,† Ksenia Timachova,‡ Michał Banaszak,*,∥,⊥ and Nitash P
Perspective pubs.acs.org/Macromolecules 50th Anniversary Perspective: Phase Behavior of Polymer Solutions and Blends Piotr Knychała,† Ksenia Timachova,‡ Michał Banaszak,*,∥,⊥ and Nitash P. Balsara*,‡,# † Faculty of Polytechnic, The President Stanislaw Wojciechowski State University of Applied Sciences, Kalisz, Poland ‡ Department of Chemical and Biomolecular Engineering, University of California, Berkeley, Berkeley, California 94720, United States ∥ ⊥ Faculty of Physics and NanoBioMedical Centre, Adam Mickiewicz University, ul. Umultowska 85, 61-614 Poznan, Poland # Materials Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, California 94720, United States *S Supporting Information ABSTRACT: We summarize our knowledge of the phase behavior of polymer solutions and blends using a unified approach. We begin with a derivation of the Flory−Huggins expression for the Gibbs free energy of mixing two chemically dissimilar polymers. The Gibbs free energy of mixing of polymer solutions is obtained as a special case. These expressions are used to interpret observed phase behavior of polymer solutions and blends. Temperature- and pressure-dependent phase diagrams are used to determine the Flory−Huggins interaction parameter, χ. We also discuss an alternative approach for measuring χ due to de Gennes, who showed that neutron scattering from concentration fluctuations in one-phase systems was a sensitive function of χ. In most cases, the agreement between experimental data and the standard Flory−Huggins−de Gennes approach is qualitative. We conclude by summarizing advanced theories that have been proposed to address the limitations of the standard approach. In spite of considerable effort, there is no consensus on the reasons for departure between the standard theories and experiments. ■ INTRODUCTION mean-field theory of de Gennes and their effect on phase 4,5 Our understanding of the thermodynamic underpinnings of the behavior. -

Caged Polyphosphazenes with a Visible‐
COMMUNICATION Photocleavable Polymers www.mrc-journal.de Coumarin-Caged Polyphosphazenes with a Visible-Light Driven On-Demand Degradation Aitziber Iturmendi, Sabrina Theis, Dominik Maderegger, Uwe Monkowius, and Ian Teasdale* desired response. Consequently, several Polymers that, upon photochemical activation with visible light, undergo photo-responsive polymers have been pre- rapid degradation to small molecules are described. Through functionaliza- pared that have great promise in diverse tion of a polyphosphazene backbone with pendant coumarin groups sensitive applications such as drug delivery, porous membranes, and film patterning.[4b,9] to light, polymers which are stable in the dark could be prepared. Upon irra- Macromole cules that undergo photo- diation, cleavage of the coumarin moieties exposes carboxylic acid moieties chemical backbone degradation are also along the polymer backbone. The subsequent macromolecular photoacid is of significant interest.[10] Most reported found to catalyze the rapid hydrolytic degradation of the polyphosphazene photo-cleavable systems require ultraviolet backbone. Water-soluble and non-water-soluble polymers are reported, which (UV) light to achieve cleavage, which can due to their sensitivity toward light in the visible region could be significant be a drawback especially in biomedical environments due to low penetration and as photocleavable materials in biological applications. biocompatibility, hence a shift to longer wavelengths is required.[11] Coumarin and its analogs are widely employed