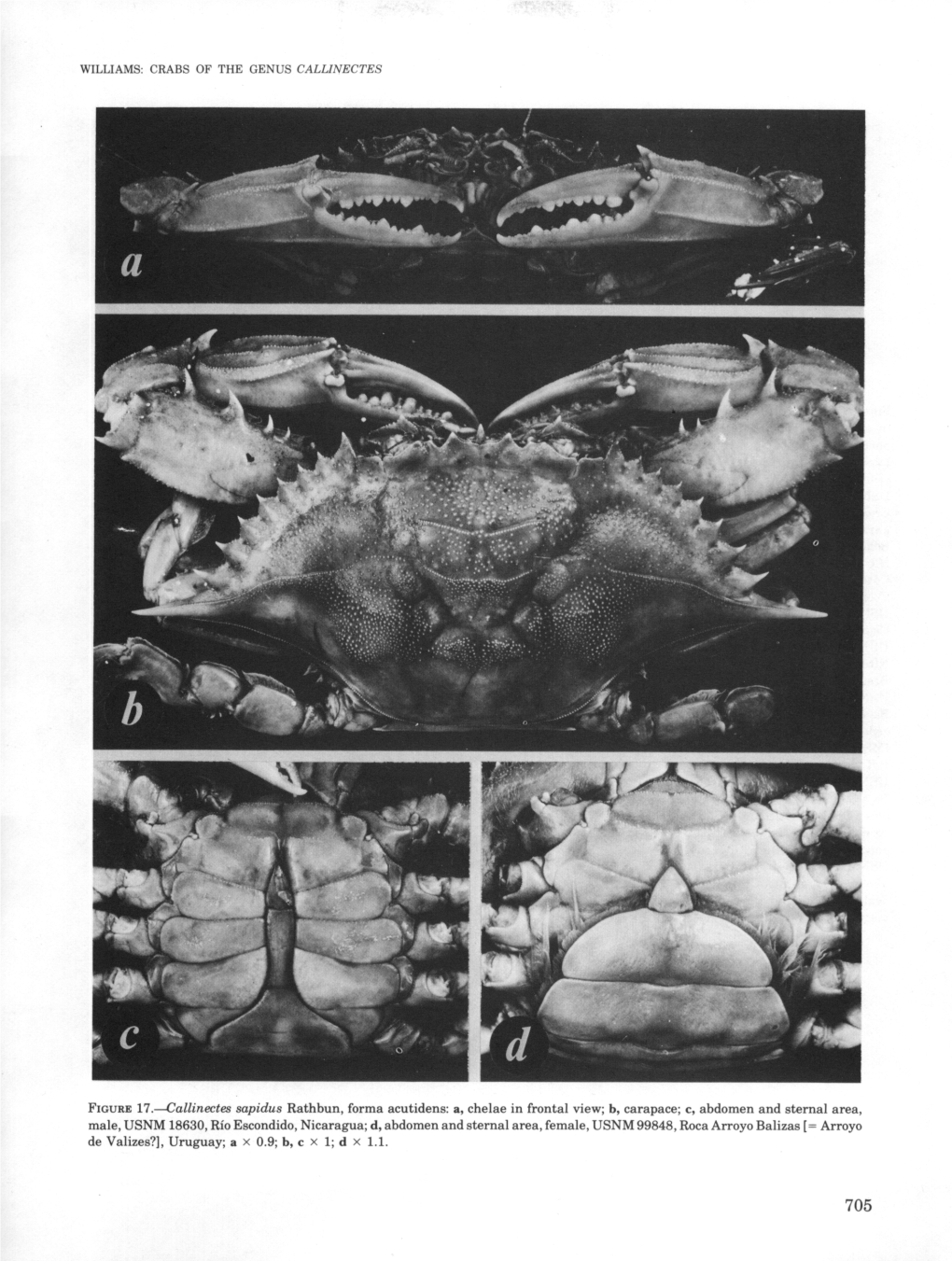
FIGURE 17.—Callinectes Sapidus Rathbun, Forma Acutidens: A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Checklists of Crustacea Decapoda from the Canary and Cape Verde Islands, with an Assessment of Macaronesian and Cape Verde Biogeographic Marine Ecoregions
Zootaxa 4413 (3): 401–448 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2018 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4413.3.1 http://zoobank.org/urn:lsid:zoobank.org:pub:2DF9255A-7C42-42DA-9F48-2BAA6DCEED7E Checklists of Crustacea Decapoda from the Canary and Cape Verde Islands, with an assessment of Macaronesian and Cape Verde biogeographic marine ecoregions JOSÉ A. GONZÁLEZ University of Las Palmas de Gran Canaria, i-UNAT, Campus de Tafira, 35017 Las Palmas de Gran Canaria, Spain. E-mail: [email protected]. ORCID iD: 0000-0001-8584-6731. Abstract The complete list of Canarian marine decapods (last update by González & Quiles 2003, popular book) currently com- prises 374 species/subspecies, grouped in 198 genera and 82 families; whereas the Cape Verdean marine decapods (now fully listed for the first time) are represented by 343 species/subspecies with 201 genera and 80 families. Due to changing environmental conditions, in the last decades many subtropical/tropical taxa have reached the coasts of the Canary Islands. Comparing the carcinofaunal composition and their biogeographic components between the Canary and Cape Verde ar- chipelagos would aid in: validating the appropriateness in separating both archipelagos into different ecoregions (Spalding et al. 2007), and understanding faunal movements between areas of benthic habitat. The consistency of both ecoregions is here compared and validated by assembling their decapod crustacean checklists, analysing their taxa composition, gath- ering their bathymetric data, and comparing their biogeographic patterns. Four main evidences (i.e. different taxa; diver- gent taxa composition; different composition of biogeographic patterns; different endemicity rates) support that separation, especially in coastal benthic decapods; and these parametres combined would be used as a valuable tool at comparing biotas from oceanic archipelagos. -

Part I. an Annotated Checklist of Extant Brachyuran Crabs of the World
THE RAFFLES BULLETIN OF ZOOLOGY 2008 17: 1–286 Date of Publication: 31 Jan.2008 © National University of Singapore SYSTEMA BRACHYURORUM: PART I. AN ANNOTATED CHECKLIST OF EXTANT BRACHYURAN CRABS OF THE WORLD Peter K. L. Ng Raffles Museum of Biodiversity Research, Department of Biological Sciences, National University of Singapore, Kent Ridge, Singapore 119260, Republic of Singapore Email: [email protected] Danièle Guinot Muséum national d'Histoire naturelle, Département Milieux et peuplements aquatiques, 61 rue Buffon, 75005 Paris, France Email: [email protected] Peter J. F. Davie Queensland Museum, PO Box 3300, South Brisbane, Queensland, Australia Email: [email protected] ABSTRACT. – An annotated checklist of the extant brachyuran crabs of the world is presented for the first time. Over 10,500 names are treated including 6,793 valid species and subspecies (with 1,907 primary synonyms), 1,271 genera and subgenera (with 393 primary synonyms), 93 families and 38 superfamilies. Nomenclatural and taxonomic problems are reviewed in detail, and many resolved. Detailed notes and references are provided where necessary. The constitution of a large number of families and superfamilies is discussed in detail, with the positions of some taxa rearranged in an attempt to form a stable base for future taxonomic studies. This is the first time the nomenclature of any large group of decapod crustaceans has been examined in such detail. KEY WORDS. – Annotated checklist, crabs of the world, Brachyura, systematics, nomenclature. CONTENTS Preamble .................................................................................. 3 Family Cymonomidae .......................................... 32 Caveats and acknowledgements ............................................... 5 Family Phyllotymolinidae .................................... 32 Introduction .............................................................................. 6 Superfamily DROMIOIDEA ..................................... 33 The higher classification of the Brachyura ........................ -

Barnegat Bay— Year 2
Plan 9: Research Barnegat Bay— Benthic Invertebrate Community Monitoring & Year 2 Indicator Development for the Barnegat Bay-Little Egg Harbor Estuary - Barnegat Bay Diatom Nutrient Inference Model Hard Clams as Indicators of Suspended Ecological Evaluation of Particulates in Barnegat Bay Sedge Island Marine Assessment of Fishes & Crabs Responses to Conservation Zone Human Alteration of Barnegat Bay Assessment of Stinging Sea Nettles (Jellyfishes) in Barnegat Bay Baseline Characterization Dr. Paul Jivoff, Rider University, Principal Investigator of Phytoplankton and Harmful Algal Blooms Project Manager: Joe Bilinski, Division of Science, Research and Environmental Health Baseline Characterization of Zooplankton in Barnegat Bay Thomas Belton, Barnegat Bay Research Coordinator Dr. Gary Buchanan, Director—Division of Science, Research & Environmental Health Multi-Trophic Level Modeling of Barnegat Bob Martin, Commissioner, NJDEP Bay Chris Christie, Governor Tidal Freshwater & Salt Marsh Wetland Studies of Changing Ecological Function & Adaptation Strategies 29 August 2014 Final Report Project Title: Ecological Evaluation of Sedge Island Marine Conservation Area in Barnegat Bay Dr. Paul Jivoff, Rider University, Manager [email protected] Joseph Bilinski, NJDEP Project Manager [email protected] Tom Belton, NJDEP Research Coordinator [email protected] Marc Ferko, NJDEP Quality Assurance Officer [email protected] Acknowledgements I would like to thank the Rutgers University Marine Field Station for providing equipment, facilities and logistical support that were vital to completing this project. I also thank Rider University students (Jade Kels, Julie McCarthy, Laura Moritzen, Amanda Young, Frank Pandolfo, Amber Barton, Pilar Ferdinando and Chelsea Tighe) who provided critical assistance in the field and laboratory. The Sedge Island Natural Resource Education Center provided key logistical support for this project. -

DINÂMICA POPULACIONAL DO SIRI-AZUL Callinectes Sapidus (RATHBUN, 1896) (CRUSTACEA: DECAPODA: PORTUNIDAE) NO BAIXO ESTUÁRIO DA LAGOA DOS PATOS, RS, BRASIL
UNIVERSIDADE FEDERAL DO RIO GRANDE PÓS-GRADUAÇÃO EM OCEANOGRAFIA BIOLÓGICA DINÂMICA POPULACIONAL DO SIRI-AZUL Callinectes sapidus (RATHBUN, 1896) (CRUSTACEA: DECAPODA: PORTUNIDAE) NO BAIXO ESTUÁRIO DA LAGOA DOS PATOS, RS, BRASIL LEONARDO SIMÕES FERREIRA Tese apresentada ao Programa de Pós- graduação em Oceanografia Biológica da Universidade Federal do Rio Grande, como requisito parcial à obtenção do título de DOUTOR. Orientador: Fernando D´Incao RIO GRANDE Janeiro/2012 AGRADECIMENTOS Em primeiro lugar ao meu amigo, professor e orientador Dr. Fernando D´Incao, por seus ensinamentos durante todos esses anos. Ao meu coorientador e amigo Dr. Duane Fonseca, por toda ajuda no decorrer da Tese, e principalmente por me passar todo o seu conhecimento sobre o assunto “lipofuscina”. Aos Doutores, Paulo Juarez Rieger, Enir Girondi Reis (Neca), Wilson Wasieleski (Mano), e Rogério Caetano (Cebola) da Unespe, por aceitarem fazer parte da minha banca examinadora, e por suas valiosas correções e sugestões. Toda a galera do Laboratório de Crustáceos Decapodes, os quais são muitos! A minha amiga especial Laboratorista/Dra. Roberta Barutot que me ajudou em grande parte da Tese, assim como o Doutor Luiz Felipe Dumont. Aos meus estagiários, Andréia Barros, Renan (bonitão.com) e Diego Martins (guasco). Meus amigos pescadores: Pingo, Sarinha, Leandro, Giovani e Didico. A minha família, meus pais, minha esposa Juliana e a minha princesinha Luana! Ao Programa de Pós-graduação em Oceanografia Biológica, a Capes pela concessão da bolsa de estudos, ao Instituto de -

Generation of Added Value Through the Process of Soft Shell Crab: a Sustainable Development Option in the Coastal Region of Sonora
Journal of Management and Sustainability; Vol. 5, No. 2; 2015 ISSN 1925-4725 E-ISSN 1925-4733 Published by Canadian Center of Science and Education Generation of Added Value through the Process of Soft Shell Crab: A Sustainable Development Option in the Coastal Region of Sonora Luis E. Ibarra1, Erika Olivas1, A. Lourdes Partida2 & Daniel Paredes3 1 School of International Trade, Sonora State University, Hermosillo, Sonora, México 2 School of English Language Teaching, Sonora State University, Hermosillo, Sonora, México 3 School of Agribusiness Management, Sonora State University, Benito Juarez, Sonora, México Correspondence: Luis E. Ibarra, School of International Trade, Sonora State University, Hermosillo, Sonora, México. Tel: 1-622-948-7708. E-mail:[email protected] or [email protected] Received: March 12, 2015 Accepted: March 30, 2015 Online Published: May 31, 2015 doi:10.5539/jms.v5n2p57 URL: http://dx.doi.org/10.5539/jms.v5n2p57 Abstract Nowadays there are fishery resources that have suffered the consequences of overexploitation, pollution or climate change, therefore, the population of marine organisms of commercial importance has diminished noticeably. One of the alternatives to mitigate this reduction, is the diversification of the fishery and aquaculture activity, through value creation.To do this, there is a great number of species to cultivate and that have not been seized due to the lack of interest or knowledge, in addition that the fishery communities have not been provided with the sufficient technology to allow its -

Dieta Natural Do Siri-Azul Callinectes Sapidus (Decapoda, Portunidae) Na Região
Dieta natural do siri-azul Callinectes sapidus (Decapoda, Portunidae) na região... 305 Dieta natural do siri-azul Callinectes sapidus (Decapoda, Portunidae) na região estuarina da Lagoa dos Patos, Rio Grande, Rio Grande do Sul, Brasil Alexandre Oliveira, Taciana K. Pinto, Débora P. D. Santos & Fernando D’Incao Fundação Universidade Federal do Rio Grande, Caixa Postal 474, 96201-900 Rio Grande, RS, Brasil. ([email protected], [email protected], [email protected], [email protected]) ABSTRACT. Natural diet of the blue crab Callinectes sapidus (Decapoda, Portunidae) in the Patos Lagoon estuary area, Rio Grande, Rio Grande do Sul, Brazil. The Southern Brazil blue crab Callinectes sapidus Rathbun, 1869 is the most abundant crab of the genus Callinectes in Patos Lagoon estuary. Although this species is widely distributed throughout the Patos Lagoon estuary area, there is little information about its natural diet. This species is an important predator and has a significant influence on its prey populations. The aim of this study was to check the natural diet of blue crab through the foregut contents analysis. Crabs were collected using an otter trawl net from March 2003 to March 2004. After collected, crabs were preserved immediately in 10% formaldehyde. The carapace width, weight and sex were measured for each individual. The foregut of each crab was removed and stored in 70% ethanol. Blue crab feeds on a wide variety of sessile and slow moving invertebrates. The main item was Detritus, followed by the suspension-feeder mollusk Erodona mactroides Bosc, 1802 (Erodonidae). Sand grains and the small crustaceans of class Ostracoda, were an important component of the foregut contents, but sand grains were not considered food. -

Revista Nicaragüense De Biodiversidad
ISSN 2413-337X REVISTA NICARAGÜENSE DE BIODIVERSIDAD N°57. Enero 2020 Depredación del ostión de mangle (Crassostrea rhizophorae) por la jaiba prieta (Callinectes rathbunae) en Tabasco, México Saúl Sánchez-Soto PUBLICACIÓN DEL MUSEO ENTOMOLÓGICO ASOCIACIÓN NICARAGÜENSE DE ENTOMOLOGÍA LEÓN - - - NICARAGUA REVISTA NICARAGÜENSE DE BIODIVERSIDAD. No. 57. 2020. La Revista Nicaragüense de Biodiversidad (ISSN 2413-337X) es una publicación que pretende apoyar a la divulgación de los trabajos realizados en Nicaragua en este tema. Todos los artículos que en ella se publican son sometidos a un sistema de doble arbitraje por especialistas en el tema. The Revista Nicaragüense de Biodiversidad (ISSN 2413-337X) is a journal created to help a better divulgation of the research in this field in Nicaragua. Two independent specialists referee all published papers. Consejo Editorial Jean Michel Maes Editor General Museo Entomológico Nicaragua Milton Salazar Eric P. van den Berghe Herpetonica, Nicaragua ZAMORANO, Honduras Editor para Herpetología. Editor para Peces. Liliana Chavarría Arnulfo Medina ALAS, El Jaguar Nicaragua Editor para Aves. Editor para Mamíferos. Oliver Komar Estela Yamileth Aguilar ZAMORANO, Honduras Álvarez Editor para Ecología. ZAMORANO, Honduras Editor para Biotecnología. Indiana Coronado Missouri Botanical Garden/ Herbario HULE-UNAN León Editor para Botánica. Foto de Portada: Macho de Callinectes rathbunae recolectado en el sistema estuarino de la laguna El Carmen, Cárdenas, Tabasco, México (Foto: Saúl Sánchez- Soto). _____________________________________ -

Diet, Feeding Habits, and Predator Size/Prey Size Relationships of Red Drum (Sciaenops Ocellatus) in Galveston Bay, Texas
Diet, Feeding Habits, and Predator Size/Prey Size Relationships of Red Drum (Sciaenops ocellatus) in Galveston Bay, Texas 233 Kurtis K. Schlicht Conservation Scientist II Texas Parks and Wildlife, Coastal Fisheries Division Seabrook Marine Laboratory B.S. Degree in Biology from Texas Tech University, Lubbock, Texas. Previous work includes Biologist Assistant for six years with Houston Lighting and Power's Environmental Division. Currently working as a Conservation Scientist II in the Coastal Fisheries Division of the Texas Parks and Wildlife, Seabrook Marine Laboratory. (281) 474-2811. 234 Feeding Habits of Red Drum (Sciaenops ocellatus) in Galveston Bay, Texas: Seasonal Diet Variation and Predator-Prey Size Relationships Frederick S. Scharf and Kurtis K. Schlicht Texas Parks and Wildlife, Coastal Fisheries Division, Seabrook Marine Laboratory, P.O. Box 8, Seabrook, TX 77586 Introduction The effect of predaceous fishes on the composition of aquatic ecosystems can be significant. Mortality induced by predation can reduce prey abundances locally and may limit prey recruitment in some systems. In order to begin to quantify the potential effects offish predation on community structure and prey populations, detailed information is needed on the feeding habits of important predators. The red drum (Sciaenops ocellatus) is an abundant estuarine-dependent fish that is widely distributed throughout the Gulf of Mexico. Adults spawn in near shore Gulf waters close to the mouths of passes and inlets during late summer and early fall. Larvae are transported through passes into estuaries via tidal currents, where they settle in shallow nursery areas and remain through the juvenile stage. Although older red drum may migrate to offshore waters during fall and winter, fish at least as old as age four commonly occur in Gulf of Mexico estuaries. -

Physiological and Biochemical Responses to Hypoxia in the Blue Crab, Callinectes Sapidus Rathbun, the Lesser Blue Crab, Callinec
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1993 Physiological and Biochemical Responses to Hypoxia in the Blue Crab, Callinectes Sapidus Rathbun, the Lesser Blue Crab, Callinectes Similis Williams, and the Southern Oyster Drill, Stramonita Haemastoma Linnaeus. Tapash Das Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Das, Tapash, "Physiological and Biochemical Responses to Hypoxia in the Blue Crab, Callinectes Sapidus Rathbun, the Lesser Blue Crab, Callinectes Similis Williams, and the Southern Oyster Drill, Stramonita Haemastoma Linnaeus." (1993). LSU Historical Dissertations and Theses. 5625. https://digitalcommons.lsu.edu/gradschool_disstheses/5625 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. -
![Genus Panopeus H. Milne Edwards, 1834 Key to Species [Based on Rathbun, 1930, and Williams, 1983] 1](https://docslib.b-cdn.net/cover/3402/genus-panopeus-h-milne-edwards-1834-key-to-species-based-on-rathbun-1930-and-williams-1983-1-1233402.webp)
Genus Panopeus H. Milne Edwards, 1834 Key to Species [Based on Rathbun, 1930, and Williams, 1983] 1
610 Family Xanthidae Genus Panopeus H. Milne Edwards, 1834 Key to species [Based on Rathbun, 1930, and Williams, 1983] 1. Dark color of immovable finger continued more or less on palm, especially in males. 2 Dark color of immovable finger not continued on palm 7 2. (1) Outer edge of fourth lateral tooth longitudinal or nearly so. P. americanus Outer edge of fourth lateral tooth arcuate 3 3. (2) Edge of front thick, beveled, and with transverse groove P. bermudensis Edge of front if thick not transversely grooved 4 4. (3) Major chela with cusps of teeth on immovable finger not reaching above imaginary straight line drawn between tip and angle at juncture of finger with anterior margin of palm (= length immovable finger) 5 Major chela with cusps of teeth near midlength of immovable finger reaching above imaginary straight line drawn between tip and angle at juncture of finger with anterior margin of palm (= length immovable finger) 6 5. (4) Coalesced anterolateral teeth 1-2 separated by shallow rounded notch, 2 broader than but not so prominent as 1; 4 curved forward as much as 3; 5 much smaller than 4, acute and hooked forward; palm with distance between crest at base of movable finger and tip of cusp lateral to base of dactylus 0.7 or less length of immovable finger P. herbstii Coalesced anterolateral teeth 1-2 separated by deep rounded notch, adjacent slopes of 1 and 2 about equal, 2 nearly as prominent as 1; 4 not curved forward as much as 3; 5 much smaller than 4, usually projecting straight anterolaterally, sometimes slightly hooked; distance between crest of palm and tip of cusp lateral to base of movable finger 0.8 or more length of immovable finger P. -

Oxygen Consumption of the Crab Callinectes Rathbunae Parasitized by the Rhizocephalan Barnacle Loxothylacus Texanus As a Function of Salinity
MARINE ECOLOGY PROGRESS SERIES Vol. 235: 189–194, 2002 Published June 19 Mar Ecol Prog Ser Oxygen consumption of the crab Callinectes rathbunae parasitized by the rhizocephalan barnacle Loxothylacus texanus as a function of salinity Rafael Robles1, 2, Fernando Alvarez1,*, Guillermina Alcaraz3 1Colección Nacional de Crustáceos, Instituto de Biología, Universidad Nacional Autónoma de México, Apartado Postal 70-153, 04510 D. F., Mexico 2Department of Biology, University of Louisiana, Lafayette, PO Box 42451, Lafayette, Louisiana 70504-2451, USA 3Laboratorio de Ecofisiología, Departamento de Biología, Facultad de Ciencias, Universidad Nacional Autónoma de México, Apartado Postal 70-371, 04510 D. F., Mexico ABSTRACT: Rhizocephalan parasitism is one of the most important biotic factors affecting commer- cially valuable crab species in families such as Portunidae and Lithodidae. In addition to the long term and permanent effects of this parasitism on the hosts (e.g. sterilization, cessation of growth), other functional problems may arise due to the considerable size of the parasite and to its particular position inside and outside of the host. In this study, experiments with the Mexican blue crab Calli- nectes rathbunae parasitized by the rhizocephalan barnacle Loxothylacus texanus were conducted in the laboratory to test whether the parasite affects the host’s oxygen consumption rate under chang- ing salinity conditions. A total of 83 crabs (49 parasitized and 34 controls), all initially acclimated to a salinity of 5, were used for metabolic rate measurements over sequential 24 h periods at salinities of 5, 15 and 25. During this 3 d period, oxygen consumption of individual crabs was measured 5 times per day. -

A Review of the Impact of Diseases on Crab and Lobster Fisheries
A Review Of The Impact Of Diseases On Crab And Lobster Fisheries Jeffrey D. Shields Virginia Institute of Marine Science, The College of William and Mary, Gloucester Point, VA 23062 Abstract Diseases are a natural component of crustacean populations. Background levels of various agents are expected in fished populations, and there is good reason to establish baseline levels of pathogens in exploited fisheries before they become a problem. Such baselines are often difficult to fund or publish; nonetheless, outbreaks are an integral feature of heavily exploited populations. Mortalities or other problems can arise when an outbreak occurs, and all too often the underlying causes of an outbreak are poorly understood. A variety of stressors can lead to outbreaks of disease or contribute to their severity. Pollution, poor water quality, hypoxia, temperature extremes, overexploitation have all been implicated in various outbreaks. This review focuses on epidemic diseases of commercially important crabs and lobsters as well as a few examples of other disease issues in crustaceans that are ecologically important, but not of commercial significance. Key words: pathogens, crustaceans, mortality, Carcinonemertes, PaV1, overfishing, Rhizo- cephala, Paramoeba Introduction Pathogens cause direct and indirect losses to fish were thought to contain presumptive crustacean fisheries. Direct losses are obvi- toxins (Magnien 2001). At the time, the ous, resulting in morbidity or mortality to scare threatened the commercial fishing in- the fished species, but they can be difficult dustry of Chesapeake Bay because consum- to assess. However, mortality events can be ers did not purchase fish from the region. widespread and can even damage the socio- economics of impacted fishing communities, Direct losses are most visible to the fishery such as the lobster mortality event in Long because the outcome is usually morbidity or Island Sound during 1999 (Pearce and mortality to the targeted component of the Balcom 2005).