A Division of the Elapid Genus Salomonelaps
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Phylogenetic Relationships of Terrestrial Australo-Papuan Elapid Snakes (Subfamily Hydrophiinae) Based on Cytochrome B and 16S Rrna Sequences J
MOLECULAR PHYLOGENETICS AND EVOLUTION Vol. 10, No. 1, August, pp. 67–81, 1998 ARTICLE NO. FY970471 Phylogenetic Relationships of Terrestrial Australo-Papuan Elapid Snakes (Subfamily Hydrophiinae) Based on Cytochrome b and 16S rRNA Sequences J. Scott Keogh,*,†,1 Richard Shine,* and Steve Donnellan† *School of Biological Sciences A08, University of Sydney, Sydney, New South Wales 2006, Australia; and †Evolutionary Biology Unit, South Australian Museum, North Terrace, Adelaide, South Australia 5000, Australia Received April 24, 1997; revised September 4, 1997 quence data support many of the conclusions reached Phylogenetic relationships among the venomous Aus- by earlier studies using other types of data, but addi- tralo-Papuan elapid snake radiation remain poorly tional information will be needed before the phylog- resolved, despite the application of diverse data sets. eny of the Australian elapids can be fully resolved. To examine phylogenetic relationships among this 1998 Academic Press enigmatic group, portions of the cytochrome b and 16S Key Words: mitochondrial DNA; cytochrome b; 16S rRNA mitochondrial DNA genes were sequenced from rRNA; reptile; snake; elapid; sea snake; Australia; New 19 of the 20 terrestrial Australian genera and 6 of the 7 Guinea; Pacific; Asia; biogeography. terrestrial Melanesian genera, plus a sea krait (Lati- cauda) and a true sea snake (Hydrelaps). These data clarify several significant issues in elapid phylogeny. First, Melanesian elapids form sister groups to Austra- INTRODUCTION lian species, indicating that the ancestors of the Austra- lian radiation came via Asia, rather than representing The diverse, cosmopolitan, and medically important a relict Gondwanan radiation. Second, the two major elapid snakes are a monophyletic clade of approxi- groups of sea snakes (sea kraits and true sea snakes) mately 300 species and 61 genera (Golay et al., 1993) represent independent invasions of the marine envi- primarily defined by their unique venom delivery sys- ronment. -

Species-Edition-Melanesian-Geo.Pdf
Nature Melanesian www.melanesiangeo.com Geo Tranquility 6 14 18 24 34 66 72 74 82 6 Herping the final frontier 42 Seahabitats and dugongs in the Lau Lagoon 10 Community-based response to protecting biodiversity in East 46 Herping the sunset islands Kwaio, Solomon Islands 50 Freshwater secrets Ocean 14 Leatherback turtle community monitoring 54 Freshwater hidden treasures 18 Monkey-faced bats and flying foxes 58 Choiseul Island: A biogeographic in the Western Solomon Islands stepping-stone for reptiles and amphibians of the Solomon Islands 22 The diversity and resilience of flying foxes to logging 64 Conservation Development 24 Feasibility studies for conserving 66 Chasing clouds Santa Cruz Ground-dove 72 Tetepare’s turtle rodeo and their 26 Network Building: Building a conservation effort network to meet local and national development aspirations in 74 Secrets of Tetepare Culture Western Province 76 Understanding plant & kastom 28 Local rangers undergo legal knowledge on Tetepare training 78 Grassroots approach to Marine 30 Propagation techniques for Tubi Management 34 Phantoms of the forest 82 Conservation in Solomon Islands: acts without actions 38 Choiseul Island: Protecting Mt Cover page The newly discovered Vangunu Maetambe to Kolombangara River Island endemic rat, Uromys vika. Image watershed credit: Velizar Simeonovski, Field Museum. wildernesssolomons.com WWW.MELANESIANGEO.COM | 3 Melanesian EDITORS NOTE Geo PRODUCTION TEAM Government Of Founder/Editor: Patrick Pikacha of the priority species listed in the Critical Ecosystem [email protected] Solomon Islands Hails Partnership Fund’s investment strategy for the East Assistant editor: Tamara Osborne Melanesian Islands. [email protected] Barana Community The Critical Ecosystem Partnership Fund (CEPF) Contributing editor: David Boseto [email protected] is designed to safeguard Earth’s most biologically rich Prepress layout: Patrick Pikacha Nature Park Initiative and threatened regions, known as biodiversity hotspots. -

Care Guide Red Eyed Crocodile Skink
Tribolonotus gracilis Care Red Eyed Guide Crocodile Skink Shy, prehistoric pets. A relatively small skink with a prehistoric appearance, the red-eyed crocodile skink is easy to care for for experienced reptile parents. While they are not incredibly active, their elusive nature and marvelous appearance are sure to inspire any reptile enthusiast fortunate enough to observe them. They do best in warm, humid, bioactive enclosures. If you are looking to add a little dinosaur to your latest terrarium build, give the red-eyed crocodile skink a try! Lifespan With proper care, these skinks have an average lifespan of 6-10+ years. Size Hatchlings measure 6-8 cm. Adults measure 6-10 inches, snout to tail. Juveniles have the same body type and skin as adults but differ in their eye and head coloration. The head of a juvenile will be cream colored and will lack the distinctive orange/red around their eyes. At around 6 months of age, the cream color will disappear, and they will develop the orange/red around their eyes. These skinks do not reach sexual maturity until 3-4 years of age, and as they mature, they develop an orange colored throat. The male is often larger than the female, but that is not always the best indicator of sex. Males have gray-blue raised pores on the underside of the third and fourth toes of their hind feet. Males also have a rectangular section of four to six enlarged belly scales at the umbilicus. Natural History There are eight known species in the genus Tribolonotus, which are all extant to Irian Jaya and Papua New Guinea in the Solomon Islands. -

A Phylogeny and Revised Classification of Squamata, Including 4161 Species of Lizards and Snakes
BMC Evolutionary Biology This Provisional PDF corresponds to the article as it appeared upon acceptance. Fully formatted PDF and full text (HTML) versions will be made available soon. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes BMC Evolutionary Biology 2013, 13:93 doi:10.1186/1471-2148-13-93 Robert Alexander Pyron ([email protected]) Frank T Burbrink ([email protected]) John J Wiens ([email protected]) ISSN 1471-2148 Article type Research article Submission date 30 January 2013 Acceptance date 19 March 2013 Publication date 29 April 2013 Article URL http://www.biomedcentral.com/1471-2148/13/93 Like all articles in BMC journals, this peer-reviewed article can be downloaded, printed and distributed freely for any purposes (see copyright notice below). Articles in BMC journals are listed in PubMed and archived at PubMed Central. For information about publishing your research in BMC journals or any BioMed Central journal, go to http://www.biomedcentral.com/info/authors/ © 2013 Pyron et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes Robert Alexander Pyron 1* * Corresponding author Email: [email protected] Frank T Burbrink 2,3 Email: [email protected] John J Wiens 4 Email: [email protected] 1 Department of Biological Sciences, The George Washington University, 2023 G St. -

Systematics of the Carlia “Fusca” Lizards (Squamata: Scincidae) of New Guinea and Nearby Islands
Systematics of the Carlia “fusca” Lizards (Squamata: Scincidae) of New Guinea and Nearby Islands George R. Zug Bishop Museum Bulletin in Zoology 5 Bishop Museum Press Honolulu, 2004 Cover: Published by Bishop Museum Press 1525 Bernice Street Honolulu, Hawai‘i 96817-2704, USA Copyright ©2004 Bishop Museum All Rights Reserved Printed in the United States of America ISSN 0893-312X Zug — Carlia “fusca” Lizards from New Guinea and Nearby Islands v TABLE OF CONTENTS Acknowledgments ......................................................................................................................... vii Abstract ........................................................................................................................................ viii Introduction ................................................................................................................................... 1 Carlia: An Analysis for Species Relationships ........................................................................... 1 Characters and Taxa .................................................................................................................. 2 Phylogenetic Analysis................................................................................................................ 8 New Guinea Carlia “fusca” ....................................................................................................... 9 Materials and Methods................................................................................................................. -

(Elapidae- Hydrophiinae), With
1 The taxonomic history of the enigmatic Papuan snake genus Toxicocalamus 2 (Elapidae: Hydrophiinae), with the description of a new species from the 3 Managalas Plateau of Oro Province, Papua New Guinea, and a revised 4 dichotomous key 5 6 Mark O’Shea1, Allen Allison2, Hinrich Kaiser3 7 8 1 Faculty of Science and Engineering, University of Wolverhampton, Wulfruna Street, 9 Wolverhampton, WV1 1LY, United Kingdom; West Midland Safari Park, Bewdley, 10 Worcestershire DY12 1LF, United Kingdom. 11 2 Department of Natural Sciences, Bishop Museum, 1525 Bernice Street, Honolulu, 12 Hawaii 96817, U.S.A. 13 3 Department of Biology, Victor Valley College, 18422 Bear Valley Road, 14 Victorville, California 92395, U.S.A.; and Department of Vertebrate Zoology, 15 National Museum of Natural History, Smithsonian Institution, Washington, DC 16 20013, U.S.A. 17 [email protected] (corresponding author) 18 Article and Review 17,262 words 19 1 20 Abstract: We trace the taxonomic history of Toxicocalamus, a poorly known genus of 21 primarily vermivorous snakes found only in New Guinea and associated island 22 archipelagos. With only a relatively limited number of specimens to examine, and the 23 distribution of those specimens across many natural history collections, it has been a 24 difficult task to assemble a complete taxonomic assessment of this group. As a 25 consequence, research on these snakes has undergone a series of fits and starts, and we 26 here present the first comprehensive chronology of the genus, beginning with its 27 original description by George Albert Boulenger in 1896. We also describe a new 28 species from the northern versant of the Owen Stanley Range, Oro Province, Papua 29 New Guinea, and we present a series of comparisons that include heretofore underused 30 characteristics, including those of unusual scale patterns, skull details, and tail tip 31 morphology. -
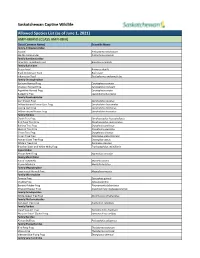
Captive Wildlife Allowed List
Saskatchewan Captive Wildlife Allowed Species List (as of June 1, 2021) AMPHIBIANS (CLASS AMPHIBIA) Class (Common Name) Scientific Name Family Ambystomatidae Axolotl Ambystoma mexicanum Marble Salamander Ambystoma opacum Family Bombinatoridae Oriental Fire-Bellied Toad Bombina orientalis Family Bufonidae Green Toad Anaxyrus debilis Black Indonesian Toad Bufo asper Indonesian Toad Duttaphrynus melanostictus Family Ceratophryidae Surinam Horned Frog Ceratophrys cornuta Chacoan Horned Frog Ceratophrys cranwelli Argentine Horned Frog Ceratophrys ornata Budgett’s Frog Lepidobatrachus laevis Family Dendrobatidae Dart Poison Frog Dendrobates auratus Yellow-banded Poison Dart Frog Dendrobates leucomelas Dyeing Dart Frog Dendrobates tinctorius Yellow-striped Poison Frog Dendrobates truncatus Family Hylidae Clown Tree Frog Dendropsophus leucophyllatus Bird Poop Tree Frog Dendropsophus marmoratus Barking Tree Frog Dryophytes gratiosus Squirrel Tree Frog Dryophytes squirellus Green Tree Frog Dryophytes cinereus Cuban Tree Frog Osteopilus septentrionalis Haitian Giant Tree Frog Osteopilus vastus White’s Tree Frog Ranoidea caerulea Brazilian Black and White Milky Frog Trachycephalus resinifictrix Hyperoliidae African Reed Frog Hyperolius concolor Family Mantellidae Baron’s Mantella Mantella baroni Brown Mantella Mantella betsileo Family Megophryidae Long-nosed Horned Frog Megophrys nasuta Family Microhylidae Tomato Frog Dyscophus guineti Chubby Frog Kaloula pulchra Banded Rubber Frog Phrynomantis bifasciatus Emerald Hopper Frog Scaphiophryne madagascariensis -

Species Richness in Time and Space: a Phylogenetic and Geographic Perspective
Species Richness in Time and Space: a Phylogenetic and Geographic Perspective by Pascal Olivier Title A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Ecology and Evolutionary Biology) in The University of Michigan 2018 Doctoral Committee: Assistant Professor and Assistant Curator Daniel Rabosky, Chair Associate Professor Johannes Foufopoulos Professor L. Lacey Knowles Assistant Professor Stephen A. Smith Pascal O Title [email protected] ORCID iD: 0000-0002-6316-0736 c Pascal O Title 2018 DEDICATION To Judge Julius Title, for always encouraging me to be inquisitive. ii ACKNOWLEDGEMENTS The research presented in this dissertation has been supported by a number of research grants from the University of Michigan and from academic societies. I thank the Society of Systematic Biologists, the Society for the Study of Evolution, and the Herpetologists League for supporting my work. I am also extremely grateful to the Rackham Graduate School, the University of Michigan Museum of Zoology C.F. Walker and Hinsdale scholarships, as well as to the Department of Ecology and Evolutionary Biology Block grants, for generously providing support throughout my PhD. Much of this research was also made possible by a Rackham Predoctoral Fellowship, and by a fellowship from the Michigan Institute for Computational Discovery and Engineering. First and foremost, I would like to thank my advisor, Dr. Dan Rabosky, for taking me on as one of his first graduate students. I have learned a tremendous amount under his guidance, and conducting research with him has been both exhilarating and inspiring. I am also grateful for his friendship and company, both in Ann Arbor and especially in the field, which have produced experiences that I will never forget. -

Presence and Absence of COX8 in Reptile Transcriptomes
Presence and Absence of COX8 in Reptile Transcriptomes Emily K. West, Michael W. Vandewege, Federico G. Hoffmann Department of Biochemistry, Molecular Biology, Entomology, and Plant Pathology Mississippi State University Mitochondria are organelles found in most eukaryotic cells that are responsible for supplying chemical energy in the form of adenosine triphosphate (ATP). Located in the mitochondria inner membrane, the electron transport chain (ETC) is series of proteins that shuttle electrons to synthesize ATP. The different components of the ETC are well conserved among eukaryotes in general and among vertebrates in particular. Previous studies have shown that COX8, a relatively small subunit of the ETC was important for optimal mitochondrial performance. Interestingly, several lizard species have apparently lost the gene encoding for this protein. The goal of this research was to assess the phylogenetic extent of the loss of COX8. To do so we looked for traces of the COX8 coding gene using bioinformatic protocols. These databases included publicly available genomes and transcriptomes, as well as several reptilian transcriptomes from our lab. Our results indicate that COX8 has apparently been lost multiple times during the evolution of amniotes, at least once among turtles, once among snakes, and once among lizards. All these losses have been recorded among ectotherm animals, suggesting the lower metabolic demands of these animals allow them to perform well in the absence of COX8. Introduction Mitochondria are organelles found in most eukaryotic cells that are responsible for supplying energy in the form of adenosine triphosphate (ATP) during aerobic respiration. The electron transport chain (ETC) is series of proteins that shuttle electrons via redox reactions, generating a proton gradient across the internal membrane of the mitochondria, with oxygen as the final electron acceptor and ATP as ultimate product (Figure 1). -

Denisonia Hydrophis Parapistocalamus Toxicocalamus Disteira Kerilia Pelamis Tropidechis Drysdalia Kolpophis Praescutata Vermicella Echiopsis Lapemis
The following is a work in progress and is intended to be a printable quick reference for the venomous snakes of the world. There are a few areas in which common names are needed and various disputes occur due to the nature of such a list, and it will of course be continually changing and updated. And nearly all species have many common names, but tried it simple and hopefully one for each will suffice. I also did not include snakes such as Heterodon ( Hognoses), mostly because I have to draw the line somewhere. Disclaimer: I am not a taxonomist, that being said, I did my best to try and put together an accurate list using every available resource. However, it must be made very clear that a list of this nature will always have disputes within, and THIS particular list is meant to reflect common usage instead of pioneering the field. I put this together at the request of several individuals new to the venomous endeavor, and after seeing some very blatant mislabels in the classifieds…I do hope it will be of some use, it prints out beautifully and I keep my personal copy in a three ring binder for quick access…I honestly thought I knew more than I did…LOL… to my surprise, I learned a lot while compiling this list and I hope you will as well when you use it…I also would like to thank the following people for their suggestions and much needed help: Dr.Wolfgang Wuster , Mark Oshea, and Dr. Brian Greg Fry. -

The Kiwi of All Skinks: an Unusual Egg Size in a Species of Madascincus (Squamata: Scincidae) from Eastern Madagascar
Herpetology Notes, volume 14: 365-369 (2021) (published online on 14 February 2021) The kiwi of all skinks: an unusual egg size in a species of Madascincus (Squamata: Scincidae) from eastern Madagascar Xavier Porcel1, Nicolas Dubos2, Gonçalo M. Rosa3, Aurélien Miralles4, Jean Nöel5, Honoré Lava5, Jean Honoré Velo5, Georges5, Franco Andreone6, and Angelica Crottini1,* In the animal world, egg, hatchling, and neonate sizes gives birth to 16.6 small neonates per litter on average are positively correlated with adult body size, although (Racey and Stephenson, 1996) with a maximum litter- small species usually produce a smaller number of size of 32 neonates (Bluntschli, 1938; Louwman, 1973), offspring per clutch in comparison to their larger the greatest number among mammals. relatives (Meiri et al., 2015). Yet, natural selection has The negative offspring-size allometry (with smaller led some species to exhibit extreme traits in this regard. species producing relatively larger offspring and Kiwis (Apteryx spp.) are the smallest of the ratite birds, larger species producing smaller offspring) is quite but their eggs are among the largest in proportion to widespread in squamates, and species showing invariant body size among all birds. The ratio between egg and clutch sizes tend to have unusually steep offspring adult body mass is one order of magnitude larger than size allometries (Meiri et al., 2015). Squamates have in other ratites, and they generally lay only one egg evolved a variety of reproductive strategies, and a (maximum two) weighing about one quarter of the lot of attention has been given to the study of their female body mass (Calder, 1979; Migeon, 2014). -

East Tennessee State University Digital Commons@ East
East Tennessee State University Digital Commons @ East Tennessee State University Electronic Theses and Dissertations Student Works 5-2011 On The rC anial Osteology of Eremiascincus and Its Use For Identification. William B. Gelnaw East Tennessee State University Follow this and additional works at: https://dc.etsu.edu/etd Part of the Paleontology Commons Recommended Citation Gelnaw, William B., "On The rC anial Osteology of Eremiascincus and Its Use For Identification." (2011). Electronic Theses and Dissertations. Paper 1294. https://dc.etsu.edu/etd/1294 This Thesis - Open Access is brought to you for free and open access by the Student Works at Digital Commons @ East Tennessee State University. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons @ East Tennessee State University. For more information, please contact [email protected]. On the Cranial Osteology of Eremiascincus, and Its Use for Identification ________________________________________ A thesis presented to the faculty of the department of Biological Sciences East Tennessee State University In partial fulfillment of the requirements for the degree Master of Sciences in Biology _______________________________________ by William B. Gelnaw May 2011 _______________________________________ James Mead, Chair Blaine Schubert Stephen Wallace Keywords: Squamata, Morphometrics, Lizards, Skull ABSTRACT On the Cranial Osteology of Eremiascincus, and Its Use for Identification by William B. Gelnaw A persistent problem