The Saga of Liver Replacement, with Particular Reference to the Reciprocal Influel1ce of Liver and Kidney Transplantation (1955-1967)
Total Page:16
File Type:pdf, Size:1020Kb
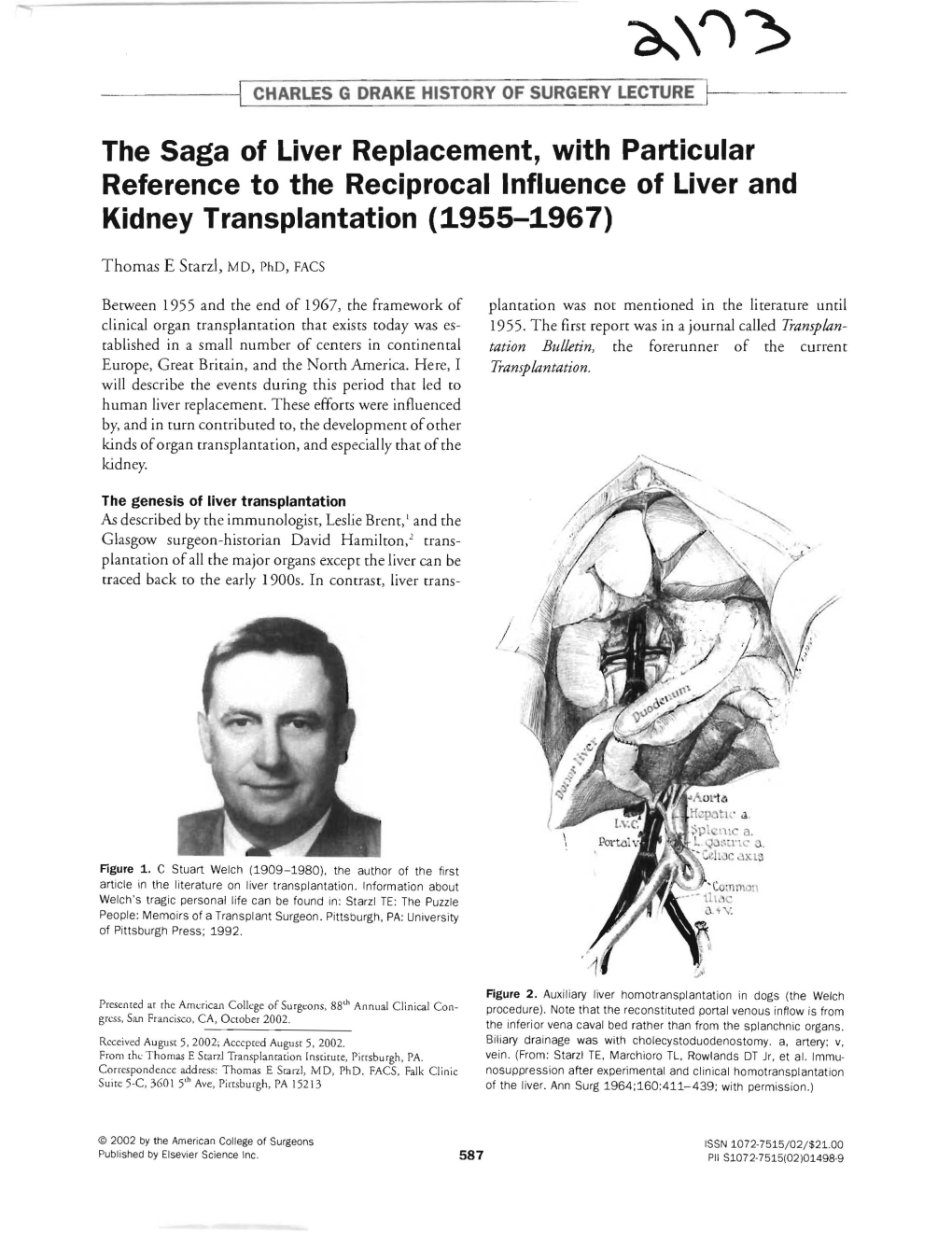
Load more
Recommended publications
-

History of Lung Transplantation Akciğer Transplantasyonu Tarihçesi
REVIEW History of Lung Transplantation Akciğer Transplantasyonu Tarihçesi Gül Dabak Unit of Pulmonology, Kartal Kosuyolu Yüksek Ihtisas Teaching Hospital for Cardiovascular Diseases and Surgery, İstanbul ABSTRACT ÖZET History of lung transplantation in the world dates back to the early 20 Dünyada akciğer transplantasyonu tarihçesi, deneysel çalışmala- th century, continues to the first clinical transplantation performed rın yapılmaya başlandığı 20. yüzyılın ilk yıllarından itibaren Ja- by James Hardy in the United States of America in 1963 and comes mes Hardy’ nin Amerika Birleşik Devletleri’nde 1963’te yaptığı to the present with increased frequency. Over 40.000 heart-lung ilk klinik transplantasyona uzanır ve hızlanarak günümüze gelir. and lung transplantations were carried out in the world up to 2011 yılına kadar dünyada 40,000’in üzerinde kalp-akciğer ve 2011. The number of transplant centers and patients is flourishing akciğer transplantasyonu yapılmıştır. Transplantasyon alanındaki in accordance with the increasing demand and success rate in that artan ihtiyaca ve başarılara paralel olarak transplant merkezleri arena. Lung transplantations that started in Turkey at Sureyyapasa ve hasta sayıları da giderek artmaktadır. Türkiye’de 2009 yılında Teaching Hospital for Pulmonary Diseases and Thoracic Surgery Süreyyapaşa Göğüs Hastalıkları ve Cerrahisi Eğitim ve Araştırma in 2009 are being performed at two centers actively to date. This Hastanesi’ nde başlayan akciğer transplantasyonları günümüzde review covers a general outlook on lung transplantations both in iki merkezde aktif olarak yapılmaktadır. Bu derlemede, ülkemiz- the world and in Turkey with details of the first successful lung deki ilk başarılı akciğer transplantasyonu detaylandırılarak dün- transplantation in our country. yada ve ülkemizdeki akciğer transplantasyonu tarihçesi gözden Keywords: Lung transplantation, heart-lung transplantation, his- geçirilmektedir. -

2004 Final Program
The International Society for Heart and Lung Transplantation (a forum that includes basic science, the failing heart, and advanced lung disease) Twenty-Fourth Annual Meeting and Scientific Sessions April 21 – 24, 2004 Convening at the Hilton San Francisco San Francisco, CA Final Program Board of Directors President Jon Kobashigawa, MD, Los Angeles, CA President-Elect Alec Patterson, MD, St. Louis, MO Past President Stephan Schueler, MD, Newcastle, United Kingdom Secretary/Treasurer Robert C. Bourge, MD, Birmingham, AL Directors Paul A. Corris, MB, FRCP, Newcastle, United Kingdom F. Jay Fricker, MD, Gainesville, FL Katherine Hoercher, RN, Cleveland, OH Luigi Martinelli, MD, Genova, Italy Keith McNeil, MD, FRACP, Brisbane, Australia Mandeep R. Mehra, MD, New Orleans, LA Soon J. Park, MD, San Francisco, CA Hermann Reichenspurner, MD, PhD, Hamburg, Germany Bruce Rosengard, MD, FRCS, FACS, Cambridge, United Kingdom Heather J. Ross, MD, Toronto, Canada Adriana Zeevi, PhD, Pittsburgh, PA JHLT Editor James K. Kirklin, MD, Birmingham, AL Heart and Lung Transplant Registry Medical Director Marshall I. Hertz, MD, Minneapolis, MN Mechanical Circulatory Support Device Database Medical Director Mario C. Deng, MD, New York, NY Staff Amanda W. Rowe Executive Director Phyllis Glenn Assistant Executive Director Director of Membership Services Lisa Edwards Director of Meetings LeeAnn Mills Director of Operations 14673 Midway Road, Suite 200 Addison, TX 75001 Phone: 972-490-9495 Fax: 972-490-9499 www.ishlt.org [email protected] PAST PRESIDENTS 1981-1982 Michael Hess, MD 1982-1984 Jack Copeland, MD 1984-1986 Terence English, FRCS 1986-1988 Stuart Jamieson, MD 1988-1990 Bruno Reichart, MD 1990-1991 Margaret Billingham, MD 1991-1992 Christian Cabrol, MD 1992-1993 John O’Connell, MD 1993-1994 Eric Rose, MD 1994-1995 John Wallwork, FRCS 1995-1996 Sharon Hunt, MD 1996-1997 William Baumgartner, MD 1997-1998 Leslie Miller, MD 1998-1999 Alan Menkis, MD, FRCS(C) 1999-2000 Robert L. -

Poczet Doktorów Honoris Causa
2016-przedtytulowa-DHC.pdf 1 4/14/16 2:49 PM 2016-tytulowe-DOKTORZY HC.pdf 1 3/23/16 11:40 AM dr n. med. Ewa Skrzypek Warszawski Uniwersytet Medyczny © Copyright by Rektor Warszawskiego Uniwersytetu Medycznego, Warszawa 2016 Źródła fotografii doktorów honoris causa: Biblioteka Główna Warszawskiego Uniwersytetu Medycznego, Dział Fotomedyczny War- szawskiego Uniwersytetu Medycznego, Dział Zbiorów Specjalnych Głównej Biblioteki Lekar- skiej w Warszawie, Katedra Historii Medycyny Collegium Medicum Uniwersytetu Jagielloń- skiego w Krakowie, Muzeum Uniwersytetu Warszawskiego, Oddział Fotografii Narodowego Archiwum Cyfrowego w Warszawie, Polska Agencja Prasowa, Zbiory Fotografii Archiwum Polskiej Akademii Nauk w Warszawie oraz zbiory własne autorki. Redakcja językowa i korekta: Daisy Miriam Skrzypek Redakcja techniczna: Agnieszka Sierakowska Projekt okładki i stron tytułowych: Maja Sosnowska ISBN: 978-83-7637-383-6 Wydanie drugie uzupełnione i rozszerzone Nakład: 500 egz. Skład i łamanie: Agnieszka Sierakowska Druk i oprawa: Paper&Tinta PRZEDMOWA Tytuł doktora honoris causa jest najwyższą godnością, nadawaną przez Senat uczelni. Statut Warszawskiego Uniwersytetu Medycznego głosi, iż do zgłaszania kandydatów do tytułu uprawnieni są członkowie Rad Wydziałów, posiadający ty- tuł profesora, a wniosek do Senatu zgłasza Rada Wydziału. Dalej Statut podaje opis procedury, która doprowadza do przyznania tej godności osobie, która po- winna wykazywać się wybitnymi osiągnięciami oraz wyróżniać się niekwestiono- wanym autorytetem i postawą moralną, bowiem tytuł honorowy doktora honoris causa jest nadawany w dowód uznania zasług w dziedzinie nauki, kultury i życia społecznego. Utrwalony niezwykle głęboko w tradycji akademickiej zwyczaj wyróżniania dok- toratem honorowym sięga swymi początkami drugiego dziesięciolecia XIX wieku. Uniwersytet Jagielloński – najstarsza polska uczelnia – nadał go po raz pierwszy w 1816 roku dwóm profesorom własnej uczelni. -

Thoracic and Cardiovascular Surgery
GREAT INSTITUTIONS One Hundred Years of History at Stanford University: Thoracic and Cardiovascular Surgery Y. Joseph Woo, MD, and Bruce A. Reitz, MD The history of thoracic and cardiovascular surgery at Stanford spans a century long period, beginning not long after the founding of Stanford University. Pioneering Stanford surgeons have made landmark discoveries and innovations in pulmonary, transplantation, thoracic aortic, mechanical circulatory support, minimally invasive, valvular, and congenital heart surgery. Fundamental research formed the foundation underlying these and many other advances. Educating and training the subsequent leaders of cardio- thoracic surgery has throughout this century-long history constituted a mission of the highest merit. New Stanford Adult Hospital Semin Thoracic Surg 27:388–397 I 2015 Elsevier Inc. All rights reserved. Central Message Keywords: History, Cardiovascular Surgery, Thoracic Surgery, Transplantation, Aortic Dissection Stanford: Upon a foundation of rigorous scien- tific investigation and dedicated teaching, Stan- ford thoracic and cardiovascular surgeons PRE-STANFORD UNIVERSITY Stanford Faculty in pioneered discoveries and innovations in pul- Lineage tracing of the history of Stanford Cardiothoracic 1914 and led the monary, transplantation, aortic, minimally inva- Surgery could be extended back to 1857, even before the Stanford surgical sive, and congenital heart surgery. founding of Stanford University. Elias Samuel Cooper, a San service at the San Francisco surgeon, authored “Report of an Operation to Francisco General Hospital2 (Fig. 2). Although he practiced a Remove a Foreign Body from Beneath the Heart” published broad spectrum of surgery, much of his clinical and experimental by the San Francisco Medico Chirurgical Association. The work and scholarly publications were in the arena of chest following year in 1858, Cooper founded the first medical surgery. -

Norman Shumway
View metadata, citation and similar papers at core.ac.uk brought to you by CORE Baumgartner et al PRESIDENTIAL BIOGRAPHY Presidentialprovided by BiographyElsevier - Publisher Connector Norman E. Shumway, MD, PhD: Visionary, innovator, humorist William A. Baumgartner, MD,a Bruce A. Reitz, MD,b Vincent L. Gott, MD,a and Sara J. Shumway, MDc Born in Kalamazoo, Michigan, in 1923, Norman Edward sent back into the infantry. He then did three quarters of Shumway, Jr, and his parents (Laura Vandervliet Shumway premed at Baylor University in Waco, Texas. and Norman Edward Shumway, Sr) moved to Jackson, When it was time for Dr Shumway to matriculate to med- Michigan, when he was 1 year of age. His parents’ business ical school, all of the military slots were filled. He took an was operating ‘‘The Home Dairy,’’ which consisted of the interim job at Western State Mental Institution in Memphis, dairy in the back section and a diner up front. He went to Tennessee, where he was an orderly for 6 months. A slot be- the local grade school and was influenced early in a potential came open at Vanderbilt University in 1945, where he career in medicine when one of his classmates died of appen- started medical school. At Vanderbilt he was influenced dicitis. At Jackson High School, Dr Shumway was active on by 2 prominent surgeons of the time: Dr Barney Brooks, the debate team. His team was highly successful and won the Chief of Surgery, and Dr Cobb Pilcher, Chief of Neurosur- Michigan state championship in his senior year and then gery. -

Newsletteralumni News of the Newyork-Presbyterian Hospital/Columbia University Department of Surgery Volume 12 Number 1 Spring 2009
NEWSLETTERAlumni News of the NewYork-Presbyterian Hospital/Columbia University Department of Surgery Volume 12 Number 1 Spring 2009 Outliers All requires several iterative improvements and sometimes a leap of faith to cross a chasm of doubt and disappointment. This process is far more comfortable and promising if it is imbued with cross- discipline participation and basic science collaboration. Eric’s early incorporation of internist Ann Marie Schmidt’s basic science group within his Department continues to be a great example of the pro- ductivity that accrues from multidiscipline melding. Several speakers explored training in and acceptance of new techniques. The private practice community led the way in training and early adoption of laparoscopic cholecystectomy, which rapidly supplanted the open operation, despite an early unacceptable inci- dence of bile duct injuries. Mini-thoracotomies arose simultaneous- ly at multiple sites and are now well accepted as viable approaches to the coronaries and interior of the heart; whereas, more than a de- cade after their introduction, video assisted lobectomies for stage I, non-small-cell lung cancer account for <10% of US lobectomies. Os- tensibly, this reluctance reflects fear of uncontrollable bleeding and not doing an adequate cancer operation, neither of which has been a problem in the hands of VATS advocates. The May 8, 2009, 9th John Jones Surgical Day was a bit of an Lesions that are generally refractory to surgical treatment, outlier because the entire day was taken up by a single program, ex- such as glioblastomas and esophageal and pancreas cancers merit cept for a short business meeting and a lovely evening dinner party. -

Transplant Updates
Heart Transplantation A Half Century of Progress April 6, 2019 David D’Alessandro, M.D. Surgical Director, Cardiac Transplantation and Mechanical Circulatory Support Disclosures • None 2 Heart Transplantation • How did we get there? • How far have we come? • Where are we going? The danger of touching the heart "Surgery of the heart has probably reached the limits set by Nature to all surgery. No method, no new discovery, can overcome the natural difficulties that attend a wound of the heart." Stephen Paget, 1896 Alexis Carrel • published his technique for the vascular anastomosis in 1902 • 1905 reported heterotopic kidney and heart transplantation in dogs • Nobel Prize in Physiology 1912 History of Cardiac History Evolution of CPB Early Challenges in Cardiac Surgery 1920s - 1950s • Multiple failed attempts at operative treatment of rheumatic mitral stenosis • Poor visualization during ASD repairs Tubbs dilator Heart Lung Machine John Gibbon 1953 Cecilia Brevolek: May 6th as the first successful truly open-heart operation performed with the use of a heart-lung machine. Norman Shumway Surg Forum 1960;11:18. James Hardy • First human cardiac transplant was a chimpanzee xenograft performed at the University of Mississippi in 1964. Operative Permit Public ridicule “…not only immoral, but amoral”. Richard Lower • 1966 Lower performed “a reverse Hardy” • Passed up an opportunity to perform a human to human transplant in 1966 due to over cautious concern about secondary incompatibility Christiaan Barnard Louis Washkansky (December 3, 1967) “My moment of truth – the moment when the enormity of it all really hit me – was just after I had taken out Washkansky’s heart. -

A Tale of Two Kidneys Overcoming the Immunological Barrier in Organ Transplantation by Roxanna Haghighat
375Focus: Illuminating Science Years of Science at Harvard A Tale of Two Kidneys Overcoming the Immunological Barrier in Organ Transplantation By Roxanna Haghighat rgan transplantation becomes from one person and implant it into Harvard Medical School (now known Oa pesonal rather than scien- a separate human being at the outset as Brigham and Women’s Hospital), WLÀFPDWWHUDWWKH'09·VRIÀFHZKHUH seemed overwhelmingly complicated 'U0XUUD\SHUIRUPHGWKHÀUVWVXF- newly-licensed drivers are faced with DQGDOPRVWOLNHVFLHQFHÀFWLRQ³DPRUH cessful transplant of a major organ, for the crucial choice of whether or not humanistic Frankenstein, perhaps. Yet, which he received the Nobel Prize in to be an organ donor. Organ dona- decades of research later and count- Physiology in Medicine in 1990. More tion carries two seemingly polarized less obstacles overcome, organ trans- VSHFLÀFDOO\LQKHSHUIRUPHGWKH LPDJHV³RQHRI DJULHYLQJIDPLO\ plantation stands as the gold standard ÀUVWUHQDOWUDQVSODQWEHWZHHQLGHQWLFDO choosing to give life to sick patients therapeutic approach for patients with twins without organ rejection result- sitting at the top of the transplant list failing organs. ing in recipient mortality. Beyond this with no other treatment options and the While transplantation remains con- successful demonstration, Dr. Murray other of the global organ black market troversial in some cultures, as it is seen sought further to link his medical ac- by which scarce tissue resources are DVFRPPRGLÀFDWLRQRI KXPDQEHLQJV FRPSOLVKPHQWWRWKHEDVLFVFLHQWLÀF smuggled across borders. Perceptions the ability to perform organ transplants principles underlying it, investigating DVLGHWUDQVSODQWDWLRQ³ZKLOHWRGD\D LVDUHPDUNDEOHVFLHQWLÀFDFKLHYHPHQW genetics, the immune system, and organ VHWRI URXWLQHVXUJLFDOSURFHGXUHV³ that is due largely to the efforts of Dr. rejection. His discoveries, both in the UHPDLQVDQLQFUHGLEOHVFLHQWLÀFIHDW Joseph E. -

Xeno Shark Tank Session “We're Close but There Are a Few Issues to Work Out”
Xeno Shark Tank Session “We’re Close but there are a few Issues to Work Out” Andrew Adams, MD, PhD Emory University Disclosure I have no relevant disclosures. CEOT 2018- Xeno Shark Tank “Are We Ready to Take the Plunge?” Xenotransplantation • Organ Shortage is the #1 Issue in Transplantation • Optimization of Donation will never fully answer the problem • Xenotransplantation has the potential to answer the organ shortage dilemma • Genetic Engineering- New Technologies have opened new possibilities • Recent Outcomes in Preclinical Models are Promising but are we ready to try it in patients? In order to see where we’re going, it is important to remember where we’ve been Early Attempts at Clinical Xenotransplantation . Early 1960’s limited options for pts with ESRD . Deceased donor kidney transplantation infancy . 23 y/o school teacher with renal failure . chimpanzee to human kidney transplant . functioned for 9 months . 5 additional xenotransplants (8-63 days) . All died of infections . Additional heart and liver xenografts Early Attempts at Clinical Xenotransplantation Reemtsma Liver Xenotransplantation Leonard Bailey Xeno Donors . Non-human primates – ethical concerns – infectious risks – limited numbers/endangered status – concordant . Pigs – ethically acceptable, food source – easier to breed large litters – similar organ size for adults The Emory – Discordant immunologic barrier Transplant Center Challenges with Xenotransplantation . Concordant vs Discordant – distinct carbohydrate expression- a-Gal Carbohydrate xenoantigens are major -

The Point System for Organ Distribution
The Point System for Organ Distribution T.E. Starzl, R. Shapiro, and L. Teperman HEN congressional hearings on transplantation were The 1984 Gore bill was passed, but the key issues so W held by Congressmen Gore and Waxman in the important to transplant surgeons were not resolved. Extra autumn of 1983, the full implications were not obvious to renal transplant procedures remained classified as experi many who testitied. Support for the proposed legislation was mental. The key provision to fund medications was removed organized by Dr Oscar Salvatierra of San Francisco. Those from the original bill, although it was restored subsequently who contributed to the Washington support of the legislation in other legislation. The organ procurement and distribution included officers of the American Society of Transplant network that already had developed unofficially to the point Surgeons, Norman Shumway on behalf of the thoracic that it was an international model found itself under siege. A surgeons, many of the procurement coordinators, and myself task force was called for to make recommendations in (TES). There were three definable objectives. One was to transplantation, including how to distribute organs equita increase the supply of organs with a small grants program bly. Data collection was called for by a separate contract. that could strengthen procurement agencies already in exis tence or stimulate the development of new programs in THE JONASSON COMMITTEE underserved areas. An almost unnoticed proviso was the Implementation of the law was forestalled temporarily by establishment of a national network for organ distribution. ddiberations of the task force, which was instructed to The second proposal was to pay for expensive medicines, such complete its work in 6 months. -

Success for Cross-Species Heart Transplants
RESEARCH NEWS & VIEWS MEDICAL RESEARCH (perfusing) a blood-based, oxygenated solution containing nutrients and hormones through the hearts at 8 oC during the procedure (Fig. 1). This change improved short-term survival Success for cross-species in four recipient baboons, but the animals died within 40 days owing to rapid, detrimental growth of the transplanted hearts. Längin and heart transplants colleagues therefore modified the pro cedure to decrease this hypertrophy, and tested the A modified protocol has enabled baboons that received transplanted pig hearts to optimized protocol in five more baboons. First, survive for more than six months. This improvement on previous efforts brings they reduced the baboons’ blood pressure pig-to-human heart transplants a step closer. See Letter p.430 to match that of pigs. Second, they gave the baboons temsirolimus — a drug that combats heart overgrowth by stifling cell proliferation8. CHRISTOPH KNOSALLA pig-to-baboon transplants have so far lasted Third, they modified the standard hormone- only 57 days7. treatment regimen. The steroid cortisone is eart failure, in which the heart cannot Längin et al. set out to extend the survival typically given to transplant recipients to aid pump blood around the body effi- of baboons receiving life-supporting heart immunosuppression, but can cause heart over- ciently, is a problem of epic propor- transplants. They based their procedure on growth in newborn babies that receive stem- Htions. The number of adults living with heart a previously described immunosuppression cell transplants9. The authors therefore tapered failure in the United States is expected1 to reach protocol6 that prevents the baboon immune cortisone treatment much more quickly than more than 8 million by 2030, and many of system from rejecting the pig hearts, and used they had for their first group of baboons, these people will die while waiting for a donor pigs that had been genetically modified to minimizing levels of the drug by three weeks organ2,3. -

A Tale of Two Surgeons Dr Suman Nazmul Hosain Department of Cardiac Surgery, Chittagong Medical College, Chittagong
History of Cardiology A Tale of Two Surgeons Dr Suman Nazmul Hosain Department of Cardiac Surgery, Chittagong Medical College, Chittagong Abstract: Key Words : Cardiac transplantation is one of the greatest medical marvels of the twentieth century. Performing this miraculous operation on 3rd December 1967, Dr. Christiaan Barnard, an unknown surgeon Cardiac from the then apartheid state of South Africa suddenly became an international celebrity. Probably transplantation, no single procedure in the history of medicine had attracted so much media and public attention. Barnard, But there were many who thought that he didn’t deserve much of this glory. A lion share of this should have gone to somebody else. Although Barnard completed the final step in the road to Shumway. transplant, it was the end product of serious research work carried out in many centers around the World. Most important was Stanford University Medical Center, Palo Alto, California USA, where Dr. Norman Edward Shumway was engaged in transplantation related research work along with his junior colleague Dr. Richard Lower. The most of the techniques used in cardiac transplantation today were actually developed by Dr. Shumway and his team. Barnard worked in the same unit with Shumway at University of Minnesota when he came to USA. He visited USA again in 1966 when he observed the works of Shumway’s research partner Dr. Richard Lower. During both of his visits he had adopted many techniques from the research work of his American counterparts and later used in his unique accomplishment. Barnard succeeded utilizing techniques developed through Shumway’s painstaking work over the years depriving Shumway much of the glory he deserved.