Hypolimnetic Oxygen Depletion Rates in Deep Lakes: Effects of Trophic State and Organic Matter Accumulation
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Different Pockmark Systems and Their Potential Importance for the Hydrological and Biogeochemical Balance of a Perialpine Lake
Different pockmark systems and their potential importance for the hydrological and biogeochemical balance of a perialpine lake Adeline N.Y. Cojean*, Maciej Bartosiewicz**, Jeremy Zimmermann*, Moritz F. Lehmann**, Katrina Kremer*** and Stefanie B. Wirth* * Centre for Hydrogeology and Geothermics, University of Neuchatel, Rue Emile-Argand 11, CH-2000 Neuchâtel ([email protected]) ** Department of Environmental Sciences, University of Basel, Bernoullistrasse 30, CH-4056 Basel ***Swiss Seismological Service (SED), ETH Zürich, Sonneggstrasse 5, CH-8006 Zürich Lacustrine pockmarks Ø Much less investigated than marine pockmark systems Ø Fluid-flow formation Ø CH4 gas ebullition => Lake Constance (Wessel 2010; Bussmann, 2011) Ø Groundwater discharge => Lake Neuchâtel (Reusch 2015; Wirth et al., in prep.) Pockmarks in Lake Thun, Switzerland Thun Lake Thun Tannmoos Fault gypsum carying bedrock Einigen Fault Zone Spiez Fabbri et al., 2017 Beatenberg Interlaken Research questions Thun Ø Are there more pockmarks in Lake Lake Thun Thun? Ø If yes, where are they? Ø What is their mechanism of formation? Spikes in electrical Taanmoos conductivity Ø What is their influence on the lake hydrological and biogeochemical Einigen budget? Fault Zone Beatenberg karst system Spiez Beatenberg Beaten Connected to Daerligen karst system Interlaken Intensive CH4 bubbling Different pockmarks systems in Lake Thun Thun Lake Thun Connection to karst system leads to groundwater discharge? Tannmoos Einigen Fault Zone Beatenberg karst system Spiez Beatenberg Beaten Daerligen -

Investigations on the Caesium–137 Household of Lake Lugano, Switzerland
Caesium–137 Household of Lake Lugano INVESTIGATIONS ON THE CAESIUM–137 HOUSEHOLD OF LAKE LUGANO, SWITZERLAND J. DRISSNER, E. KLEMT*), T. KLENK, R. MILLER, G. ZIBOLD FH Ravensburg Weingarten, University of Applied Sciences, Center of Radioecology, P. O. Box 1261, D 88241 Weingarten, Germany M. BURGER, A. JAKOB GR, AC Laboratorium Spiez, Sektion Sicherheitsfragen, Zentrale Analytik und Radiochemie, CH 3700 Spiez, Switzerland *) [email protected] SedimentCaesium–137J. Drissner, E. HouseholdKlemt, TH. of Klenk Lake et Lugano al. samples were taken from different basins of Lake Lugano, and the caesium 137 inventory and vertical distribution was measured. In all samples, a distinct maximum at a depth of 5 to 10 cm can be attributed to the 1986 Chernobyl fallout. Relatively high specific activities of 500 to 1,000 Bq/kg can still be found in the top layer of the sediment. 5 step extraction experiments on sediment samples resulted in percentages of extracted caesium which are a factor of 2 to 8 higher than those of Lake Constance, where caesium is strongly bound to illites. The activity concentration of the water of 3 main tributaries, of the outflow, and of the lake water was in the order of 5 to 10 mBq/l. 1 Introduction Lake Lugano with an area of 48.9 km2 and a mean depth of 134 m is one of the large drinking water reservoirs of southern Switzerland, in the foothills of the southern Alps. The initial fallout of Chernobyl caesium onto the lake was about 22,000 Bq/m2 [1], which is similar to the initial fallout of about 17, 000 Bq/m2 onto Lake Constance, which is located in the prealpine area of southern Germany (north of the Alps). -

Human Impact on the Transport of Terrigenous and Anthropogenic Elements to Peri-Alpine Lakes (Switzerland) Over the Last Decades
Aquat Sci (2013) 75:413–424 DOI 10.1007/s00027-013-0287-6 Aquatic Sciences RESEARCH ARTICLE Human impact on the transport of terrigenous and anthropogenic elements to peri-alpine lakes (Switzerland) over the last decades Florian Thevenon • Stefanie B. Wirth • Marian Fujak • John Pote´ • Ste´phanie Girardclos Received: 22 August 2012 / Accepted: 6 February 2013 / Published online: 22 February 2013 Ó The Author(s) 2013. This article is published with open access at Springerlink.com Abstract Terrigenous (Sc, Fe, K, Mg, Al, Ti) and suspended sediment load at a regional scale. In fact, the anthropogenic (Pb and Cu) element fluxes were measured extensive river damming that occurred in the upstream in a new sediment core from Lake Biel (Switzerland) and watershed catchment (between ca. 1930 and 1950 and up to in previously well-documented cores from two upstream 2,300 m a.s.l.) and that significantly modified seasonal lakes (Lake Brienz and Lake Thun). These three large peri- suspended sediment loads and riverine water discharge alpine lakes are connected by the Aare River, which is the patterns to downstream lakes noticeably diminished the main tributary to the High Rhine River. Major and trace long-range transport of (fine) terrigenous particles by the element analysis of the sediment cores by inductively Aare River. Concerning the transport of anthropogenic coupled plasma mass spectrometry (ICP-MS) shows that pollutants, the lowest lead enrichment factors (EFs Pb) the site of Lake Brienz receives three times more terrige- were measured in the upstream course of the Aare River at nous elements than the two other studied sites, given by the the site of Lake Brienz, whereas the metal pollution was role of Lake Brienz as the first major sediment sink located highest in downstream Lake Biel, with the maximum val- in the foothills of the Alps. -

Constitution of an Automized Processing Chain to Analyse a Meris Time Series of Swiss Lakes
CONSTITUTION OF AN AUTOMIZED PROCESSING CHAIN TO ANALYSE A MERIS TIME SERIES OF SWISS LAKES Daniel Odermatt a, *, Thomas Heege b, Jens Nieke a, Mathias Kneubühler a and Klaus Itten a a Remote Sensing Laboratories (RSL), Dept. of Geography, University of Zurich, Winterthurerstrasse 190, CH-8050 Zurich, Switzerland - (dodermat, nieke, kneub, itten)@geo.unizh.ch b EOMAP GmbH & Co. KG, Sonderflughafen Oberpfaffenhofen, D-82205 Gilching, Germany - [email protected] KEY WORDS: Inland water, Case II water, lakes, MERIS, water constituents ABSTRACT: The physically based Modular Inversion & Processing System (MIP) is used in an automized processing chain for inland water constituent retrieval from MERIS level 1B data. Preprocessing routines are used to automatically convert the ESA generic data products into MIP input data format. Water/land masking, atmospheric correction and water constituent retrieval are accomplished by simple batch executables from MIP. The accuracy of the constituent retrieval mainly depends on the spectral fit between the image input data and the radiative transfer model results extracted from a database. Therefore, thresholds and initial values for model fitting have to account for all occurring lake specific temporal variations and need careful adjustment. 1. INTRODUCTION Heege, 2000), using the Modular Inversion & Processing System (MIP) (Heege and Fischer, 2004). Monitoring of water quality in lakes is required as an integral part of water resource management, in order to guarantee the In this work, a processing chain to automatically derive water sustainable use of water and to track the effects of constituents from MERIS level 1B data for Swiss lakes is built anthropogenic influences. Simultaneously, adequate monitoring around MIP. -

Around the Lake Constance in One Week Arrival
Around the Lake Constance in one week Individual tour Category: bike tour Duration: 7 days Offer: 2021 Meals: breakfasts Accommodation: hotels and pensions, rooms with bathrooms Difficulty: easy Distance by bike: 230 - 250 km The tour around Lake Constance is one of cycling classics. It is among the biggest lakes of Central Europe, along the Balaton and Lake Geneva. It is located at the border of Switzerland, Germany and Austria. At the same time, it is a very interestingly located one, surrounded by the Alpine mountains, allowing to observe many peaks, and numerous castles if the weather allows. The lake is encircled by a cycling route, making the trip a safe and comfortable one. Due to minor elevations we recommend the tour even for beginners and families with children. Arrival: By plane – the easiest way is coming by plane to Friedrichshafen, Stuttgart or Zurich. Most flights can be booked to Zurich, often at a reasonable price. From there, one should take a train to Constance. Places to see: Constance Over 600 years ago, the ecumenical council ending the Western schism took place in Constande. During the council, pope Martin V was elected, and the Czech reformer Jan Hus was burned at the stake at the same time. The statue of Imperia by Peter Lenk commemorates these events. Erected in 1993, weighing over 18 tons and 9 meters tall is one of the city’s symbols. It resembles a courtesan with her hands risen up, holding two naked men. One of them is wearing a crown and holds a sphere – the symbol of Holy Roman Empire. -

The Periodicity of Phytoplankton in Lake Constance (Bodensee) in Comparison to Other Deep Lakes of Central Europe
Hydrobiologia 138: 1-7, (1986). 1 © Dr W. Junk Publishers, Dordrecht - Printed in the Netherlands. The periodicity of phytoplankton in Lake Constance (Bodensee) in comparison to other deep lakes of central Europe Ulrich Sommer University of Constance, Institute of Limnology, PO. Box 5560, D-7750 Constance, FRG New address: Max Planck Institute of Limnology, PO. Box 165, D-2320 Plon, FRG Keywords: phytoplankton succession, inter-lake comparison, oligotrophic-eutrophic gradient, central Eu- ropean lakes Abstract Phytoplankton periodicity has been fairly regular during the years 1979 to 1982 in Lake Constance. Algal mass growth starts with the vernal onset of stratification; Cryptophyceae and small centric diatoms are the dominant algae of the spring bloom. In June grazing by zooplankton leads to a 'clear-water phase' dominated by Cryptophyceae. Algal summer growth starts under nutrient-saturated conditions with a dominance of Cryptomonas spp. and Pandorinamorum. Depletion of soluble reactive phosphorus is followed by a domi- nance of pennate and filamentous centric diatoms, which are replaced by Ceratium hirundinella when dis- solved silicate becomes depleted. Under calm conditions there is a diverse late-summer plankton dominated by Cyanophyceae and Dinobryon spp.; more turbulent conditions and silicon resupply enable a second sum- mer diatom growth phase in August. The autumnal development leads from a Mougeotia - desmid assem- blage to a diatom plankton in late autumn and winter. Inter-lake comparison of algal seasonality includes in ascending order of P-richness K6nigsee, Attersee, Walensee, Lake Lucerne, Lago Maggiore, Ammersee, Lake Ziirich, Lake Geneva, Lake Constance. The oligo- trophic lakes have one or two annual maxima of biomass; after the vernal maximum there is a slowly develop- ing summer depression and sometimes a second maximum in autumn. -

Long-Term Changes in the Water Level of Lake Constance and Possible Causes
Hydrology of Natural and Manmade Lakes (Proceedings of the Vienna Symposium, August 1991). IAHS Publ. no. 206, 1991. Long-term changes in the water level of Lake Constance and possible causes G. LUFT Landesanstalt fur Umweltschutz Baden-Wiirttemberg, Abt. 4: Wasser, Benzstrafie 5, D-7500 Karlsruhe 21 (F.R.G.) G. VAN DEN EERTWEGH Agricultural University Wageningen, Department of Hydrology, Soil Physics, and Hydraulics, NL-6709 PA Wageningen, formerly Landesanstalt fur Umweltschutz Baden-Wiirttemberg ABSTRACT Lake Constance is a natural reservoir system consisting of two parts, Obersee and Untersee. River Rhine flows through the lake. At the lakeside and in shallow shore zones, erosion and vegeta tion damages have been observed. Long-term changes in the water-level can be one of the causes of erosion processes and were investigated in this study. Also possible causes of these changes were taken into consideration. The results of frequency and trend analysis and Gaussian low pass filtering show that the water level of Lake Constance and its regime has changed. Mean annual water levels (Untersee), discharges (Alpenrhein and Hochrhein), and areal precipitation depths have remained nearly constant. Peak water levels and discharges have dropped, low water levels and discharges have increased. On the contrary, the mean annual water level of Obersee has dropped, low water levels have remained constant. The changes in water level have probably been caused by changes of hydraulic conditions in the outflow-regions of the lake. This process has been superposed by development and operation of storage reservoirs (hydropower purposes) in the catchment area of the Alpenrhein. Its seasonal runoff regime has been strongly influenced by an increase of low discharge, predominantly in the winter and a decrease of peak discharge in the summer. -

Neuschwanstein Castle / Füssen / Cycle Around Lake Forggensee After Breakfast, Ride to the Nearby Village of Hohenschwangau
VBT Itinerary by VBT www.vbt.com Germany, Austria & Switzerland: Bavarian Alps & Lake Constance Bike Vacation + Air Package Shimmering lakes and soaring alpine peaks set the stage for a soul-stirring bike tour of Germany, Switzerland and Austria. Immerse yourself in the fairytale castle of Neuschwanstein. You’ll cycle into charming Bavarian villages that hold historic treasures, passing wildflower meadows, dairy country, fragrant forests, and pristine waterfalls on an easy route from Füssen to Constance. Ride the invigorating downhill stretches of the scenic Bodensee-Königssee Bike Route, coasting into an intimate home-hosted lunch with ample generosity and warmth. Marvel at the baroque splendor of St. Gallen’s Abbey. Farm- fresh cuisine – organic and freshly picked – fuels your days. Austrian wines from famed wineries and renowned beers from local brewers flavor your nights. And centrally located hotels place you at the heart of historic Old Towns and picturesque parklands. Cultural Highlights 1 / 10 VBT Itinerary by VBT www.vbt.com Marvel at the three stunning crystalline lakes around Füssen – Forggensee, Alpsee and Schwansee Immerse yourself in the magical world of King Ludwig II’s enchanting Neuschwanstein Castle during an included onsite audio tour Cycle to Lindau Island on Lake Constance via the Bodensee-Königssee Bike Route, a mostly downhill spin through magnificent alpine landscapes Breathe fresh alpine air in the Bregenz Forest as you pass through flowering gardens and historic chalet villages Enjoy a lunch of local delicacies -

Active Faulting in Lake Constance (Austria, Germany, Switzerland) Unraveled by Multi-Vintage Reflection Seismic Data
ORIGINAL RESEARCH published: 11 August 2021 doi: 10.3389/feart.2021.670532 Active Faulting in Lake Constance (Austria, Germany, Switzerland) Unraveled by Multi-Vintage Reflection Seismic Data S.C. Fabbri 1*, C. Affentranger 1, S. Krastel 2, K. Lindhorst 2, M. Wessels 3, Herfried Madritsch 4, R. Allenbach 5, M. Herwegh 1, S. Heuberger 6, U. Wielandt-Schuster 7, H. Pomella 8, T. Schwestermann 8 and F.S. Anselmetti 1 1Institute of Geological Sciences and Oeschger Centre of Climate Change Research, University of Bern, Bern, Switzerland, 2Institute of Geosciences, Christian-Albrechts-Universität zu Kiel, Kiel, Germany, 3Institut für Seenforschung der LUBW, Langenargen, Germany, 4National Cooperative for the Disposal of Radioactive Waste (NAGRA), Wettingen, Switzerland, 5Federal Office of Topography Swisstopo, Wabern, Switzerland, 6Department of Earth Sciences, ETH Zürich, Zürich, Switzerland, 7Landesamt für Geologie, Rohstoffe und Bergbau Baden-Württemberg, Freiburg i. Br., Germany, 8Department of Geology, University of Innsbruck, Innsbruck, Austria Probabilistic seismic hazard assessments are primarily based on instrumentally recorded Edited by: and historically documented earthquakes. For the northern part of the European Alpine Francesco Emanuele Maesano, Istituto Nazionale di Geofisicae Arc, slow crustal deformation results in low earthquake recurrence rates and brings up the Vulcanologia (INGV), Italy necessity to extend our perspective beyond the existing earthquake catalog. The Reviewed by: overdeepened basin of Lake Constance (Austria, Germany, and Switzerland), located Chiara Amadori, within the North-Alpine Molasse Basin, is investigated as an ideal (neo-) tectonic archive. University of Pavia, Italy Alessandro Maria Michetti, The lake is surrounded by major tectonic structures and constrained via the North Alpine University of Insubria, Italy Front in the South, the Jura fold-and-thrust belt in the West, and the Hegau-Lake *Correspondence: Constance Graben System in the North. -

Managing Upper Lake Constance Fishery in a Multi- Sector Policy Landscape: Beneficiary and Victim of a Century of Anthropogenic Trophic Change
Managing Upper Lake Constance Fishery in a Multi- Sector Policy Landscape: Beneficiary and Victim of a Century of Anthropogenic Trophic Change Jan Baer1, Reiner Eckmann2, Roland Rösch1, Robert Arlinghaus3 & Alexander Brinker1 1Fisheries Research Station, Langenargen, Germany, [email protected] 2University of Konstanz, Germany 3Faculty of Life Sciences, Humboldt-Universität zu Berlin & Department of Biology and Ecology of Fishes, Leibniz-Institute of Freshwater Ecology and Inland Fisheries, Germany Abstract Upper Lake Constance (ULC) is a large pre-alpine lake situated between Austria, Germany and Switzerland (9°18'E, 47°39'N). Along with the smaller, conjoined expanse of Lower Lake Constance, it forms the third largest lake in Europe. Its waters underwent pronounced eutrophication during the 20th century. Commercial fisheries benefitted strongly from the increased productivity during an initial mesotrophic phase, but these advantages were effectively neutralized when eutrophication became severe. By the turn of the 21st century, internationally coordinated measures to reduce nutrient input to the lake had returned ULC to its historic reference state as an oligotrophic ecosystem. However, the remarkable success of the nutrient management program has been to the detriment of commercial fishers. Yields of most commercially important fish species have decreased, along with lake productivity. As a consequence, the high market demand for local fish products is nowadays met mainly by imports, the ecological footprint of which offsets the local benefits of environmental restoration. Responsibility for fisheries and environmental aspects of ULC managing is shared by the national and federal state administrations and in all cases, tourism, drinking water and environmental interests now take priority over fisheries. -

Trip Description Rhine Falls and Lake Constance by Bike
Trip description Rhine Falls and Lake Constance by bike Lake Constance is one of the most beautiful cycling destinations in Europe. Destination Europe Location Constance Lake Duration 8 day s Dif f iculty Lev el Very easy Validity f rom 24.03 to 24.10.2018 M inimum age 1 y ear Ref erence LC0801 Ty pe of stay loop trip Itinerary Lake Constance is located North of the Alps at the border between Germany, Switzerland and Austria. The lake is fed mainly by the Rhine, which falls into the lake forming the highest waterfalls in Europe. On bike paths you wind along Lake Constance, cross the fishing villages, admire the painted frescoes on the medieval houses and enjoy the small beaches of the lake for refreshing swimming stops. Take the time to visit the charming towns of Konstanz, Meersburg, Lindau, Bregenz or Arbon. Discover the open-air opera house on Lake Constance in Bregenz, enjoy an ice-cream parlor on the charming Lindau harbor and be impressed by the Rhine Falls. Day 1 Arrival in Constance Arrival in this beautiful city. Walk around the beautiful old city of Constance. Day 2 Constance - Überlingen Continue your bike ride through the German countryside beyond Constance to the Island of Mainau, the island of flowers. You reach the empire city Radolfzell and continue along Bike the lake until Überlingen. env. 50km Day 3 Überlingen - Region Bike through the vineyards until Friedrichshafen. On the way, do not miss the delightful Kressbronn baroque church of Birnau, typical for this region, the prehistoric museum in Unteruhldingen, the mediaeval city of Meersburg and its castle. -
Print Itinerary
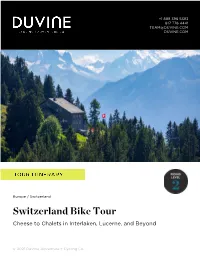
+1 888 396 5383 617 776 4441 [email protected] DUVINE.COM Europe / Switzerland Switzerland Bike Tour Cheese to Chalets in Interlaken, Lucerne, and Beyond © 2021 DuVine Adventure + Cycling Co. Sample Swiss cheese and chocolate right where it’s made Experience the abundance of outdoor landmarks in Interlaken and the jet-set appeal of Gstaad Conquer the Brünig Pass, which links the cities of Interlaken and Lucerne Cycle the shores of Lake Lucerne through quaint communities, Alpine pastures, and vineyards of Riesling and Sauvignon Blanc Arrival Details Departure Details Airport City: Airport City: Geneva, Switzerland Zurich, Switzerland Pick-Up Location: Drop-Off Location: Aigle Train Station Lucerne Train Station Pick-Up Time: Drop-Off Time: 11:30 am 11:00 am NOTE: DuVine provides group transfers to and from the tour, within reason and in accordance with the pick-up and drop-off recommendations. In the event your train, flight, or other travel falls outside the recommended departure or arrival time or location, you may be responsible for extra costs incurred in arranging a separate transfer. Emergency Assistance For urgent assistance on your way to tour or while on tour, please always contact your guides first. You may also contact the Boston office during business hours at +1 617 776 4441 or [email protected]. Travelers Take Note Hiking shoes are recommended for the hike on this tour. We suggest hiking boots or trail shoes with good traction. You may also find moisture-wicking clothing or a daypack useful, but not required. Tour By Day DAY 1 A Very Swiss Beginning Your DuVine guides will greet you in Aigle for a transfer to L'Etivaz, where our tour begins.