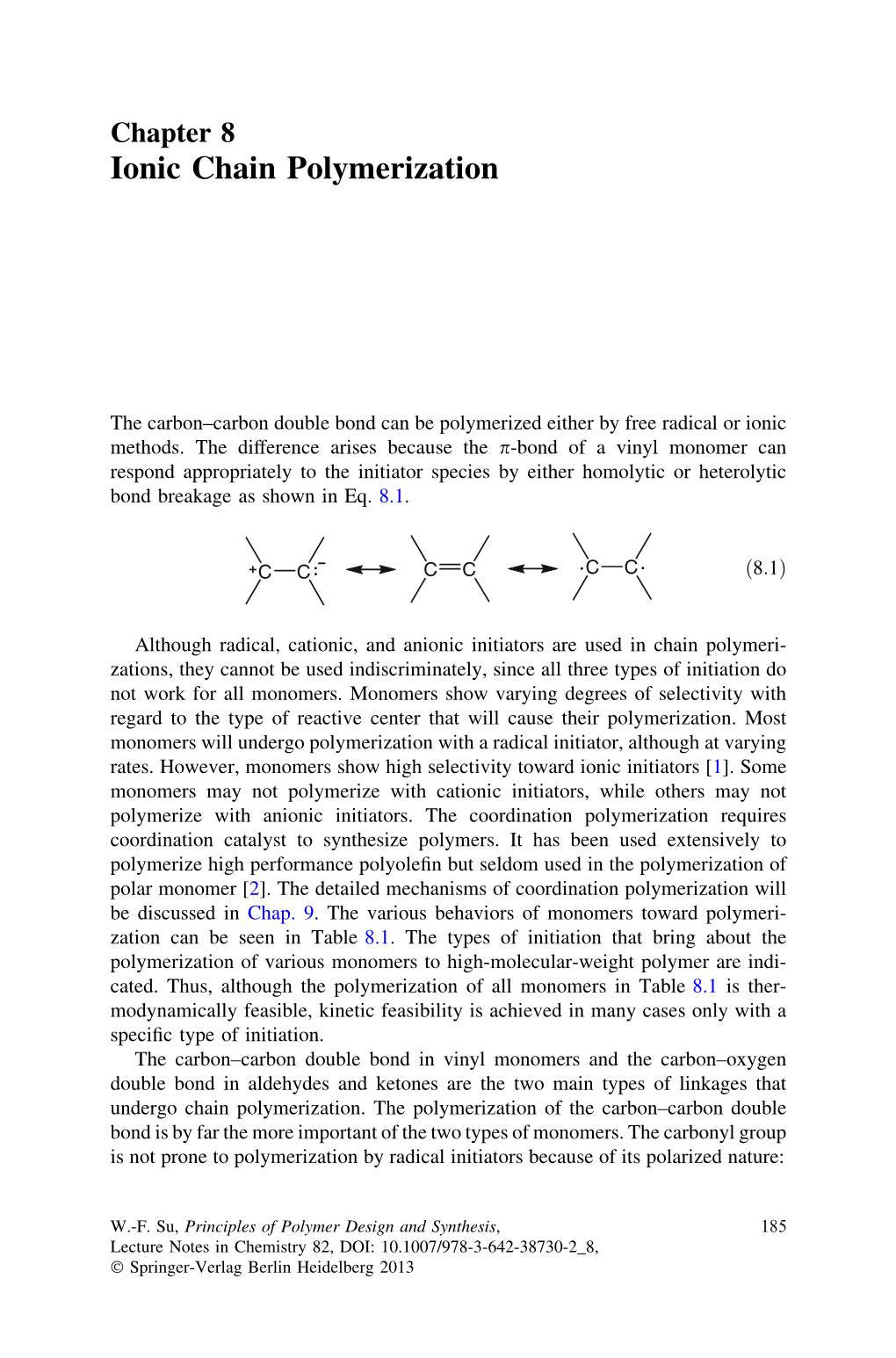
Chapter 8 Ionic Chain Polymerization
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cationic/Anionic/Living Polymerizationspolymerizations Objectives
Chemical Engineering 160/260 Polymer Science and Engineering LectureLecture 1919 -- Cationic/Anionic/LivingCationic/Anionic/Living PolymerizationsPolymerizations Objectives • To compare and contrast cationic and anionic mechanisms for polymerization, with reference to free radical polymerization as the most common route to high polymer. • To emphasize the importance of stabilization of the charged reactive center on the growing chain. • To develop expressions for the average degree of polymerization and molecular weight distribution for anionic polymerization. • To introduce the concept of a “living” polymerization. • To emphasize the utility of anionic and living polymerizations in the synthesis of block copolymers. Effect of Substituents on Chain Mechanism Monomer Radical Anionic Cationic Hetero. Ethylene + - + + Propylene - - - + 1-Butene - - - + Isobutene - - + - 1,3-Butadiene + + - + Isoprene + + - + Styrene + + + + Vinyl chloride + - - + Acrylonitrile + + - + Methacrylate + + - + esters • Almost all substituents allow resonance delocalization. • Electron-withdrawing substituents lead to anionic mechanism. • Electron-donating substituents lead to cationic mechanism. Overview of Ionic Polymerization: Selectivity • Ionic polymerizations are more selective than radical processes due to strict requirements for stabilization of ionic propagating species. Cationic: limited to monomers with electron- donating groups R1 | RO- _ CH =CH- CH =C 2 2 | R2 Anionic: limited to monomers with electron- withdrawing groups O O || || _ -C≡N -C-OR -C- Overview of Ionic Chain Polymerization: Counterions • A counterion is present in both anionic and cationic polymerizations, yielding ion pairs, not free ions. Cationic:~~~C+(X-) Anionic: ~~~C-(M+) • There will be similar effects of counterion and solvent on the rate, stereochemistry, and copolymerization for both cationic and anionic polymerization. • Formation of relatively stable ions is necessary in order to have reasonable lifetimes for propagation. -

Poly(Ethylene Oxide-Co-Tetrahydrofuran) and Poly(Propylene Oxide-Co-Tetrahydrofuran): Synthesis and Thermal Degradation
Revue Roumaine de Chimie, 2006, 51(7-8), 781–793 Dedicated to the memory of Professor Mircea D. Banciu (1941–2005) POLY(ETHYLENE OXIDE-CO-TETRAHYDROFURAN) AND POLY(PROPYLENE OXIDE-CO-TETRAHYDROFURAN): SYNTHESIS AND THERMAL DEGRADATION Thomas HÖVETBORN, Markus HÖLSCHER, Helmut KEUL∗ and Hartwig HÖCKER Lehrstuhl für Textilchemie und Makromolekulare Chemie der Rheinisch-Westfälischen Technischen Hochschule Aachen, Pauwelsstr. 8, 52056 Aachen, Germany Received January 12, 2006 Copolymers of tetrahydrofuran (THF) and ethylene oxide (EO) (poly(THF-co-EO) and THF and propylene oxide (PO) (poly(THF-co-PO) were obtained by cationic ring opening polymerization of the monomer mixture at 0°C using boron trifluoride etherate (BF3.OEt2) as the initiator. From time conversion plots it was concluded that both monomers are consumed from the very beginning of the reaction and random copolymers are obtained. For poly(THF-co-PO) the molar ratio of repeating units was varied from [THF]/[PO] = 1 to 10; the molar ratio of monomers in the feed corresponds to the molar ratio of repeating units in the copolymer. Thermogravimetric analysis of the copolymers revealed that both poly(THF-co-EO) and poly(THF-co-PO) decompose by ca. 50°C lower than poly(THF) and by ca. 100°C lower than poly(EO); 50% mass loss is obtained at T50 = 375°C for poly(EO), T50 = 330°C for poly(THF) and at T50 = 280°C for both copolymers. The [THF]/[PO] ratio does not influence the decomposition temperature significantly as well. For the copolymers the activation energies of the thermal decomposition (Ea) were determined experimentally from TGA measurements and by density functional calculations on model compounds on the B3LYP/6-31+G* level of theory. -

Importance of Sulfate Radical Anion Formation and Chemistry in Heterogeneous OH Oxidation of Sodium Methyl Sulfate, the Smallest Organosulfate
Atmos. Chem. Phys., 18, 2809–2820, 2018 https://doi.org/10.5194/acp-18-2809-2018 © Author(s) 2018. This work is distributed under the Creative Commons Attribution 4.0 License. Importance of sulfate radical anion formation and chemistry in heterogeneous OH oxidation of sodium methyl sulfate, the smallest organosulfate Kai Chung Kwong1, Man Mei Chim1, James F. Davies2,a, Kevin R. Wilson2, and Man Nin Chan1,3 1Earth System Science Programme, Faculty of Science, The Chinese University of Hong Kong, Hong Kong, China 2Chemical Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA, USA 3The Institute of Environment, Energy and Sustainability, The Chinese University of Hong Kong, Hong Kong, China anow at: Department of Chemistry, University of California Riverside, Riverside, CA, USA Correspondence: Man Nin Chan ([email protected]) Received: 30 September 2017 – Discussion started: 24 October 2017 Revised: 18 January 2018 – Accepted: 22 January 2018 – Published: 27 February 2018 Abstract. Organosulfates are important organosulfur 40 % of sodium methyl sulfate is being oxidized at the compounds present in atmospheric particles. While the maximum OH exposure (1.27 × 1012 molecule cm−3 s), only abundance, composition, and formation mechanisms of a 3 % decrease in particle diameter is observed. This can organosulfates have been extensively investigated, it remains be attributed to a small fraction of particle mass lost via unclear how they transform and evolve throughout their the formation and volatilization of formaldehyde. Overall, atmospheric lifetime. To acquire a fundamental under- we firstly demonstrate that the heterogeneous OH oxidation standing of how organosulfates chemically transform in of an organosulfate can lead to the formation of sulfate the atmosphere, this work investigates the heterogeneous radical anion and produce inorganic sulfate. -

Cationic Oligomerization of Ethylene Oxide
Polymer Journal, Vol. 15, No. 12, pp 883-889 (1983) Cationic Oligomerization of Ethylene Oxide Shiro KOBAYASHI, Takatoshi KOBAYASHI, and Takeo SAEGUSA Department of Synthetic Chemistry, Faculty of Engineering, Kyoto University, Kyoto 606, Japan (Received July 29, 1983) ABSTRACT: The oligomerization of ethylene oxide (EO) was investigated with several cationic catalysts to find a method for producing cyclic oligomers (crown ethers). The reactions were monitored by NMR spectroscopy (1 H and 19F) and gas chromatography. The composition of oligomers was found to change during the reactions, and the production of 1,4-dioxane increased at the later stage of the reactions. This indicates that the composition of oligomers is kinetically controlled. The formation of higher cyclic oligomers was favored by the addition of tetrahydro pyran or 1,4-dioxane to the system catalyzed by an oxonium salt; the maximum yield of cyclic tetramer was 16.8%. The effect of alkali metal salts was also examined. A template effect was observed to increase the amount of cyclic oligomer production. KEY WORDS Cationic Oligomerization I Ethylene Oxide I Cyclic Oligomers I Crown Ether I Kinetically Controlled Reaction I Additive Effects of Metal Salts I Template Effect I An extensive study was recently carried out on EXPERIMENTAL the cationic. polymerization of heterocyclic mono mers which, it was found, may lead to polymers Materials containing significant amounts of linear and cyclic All reagents were distilled under nitrogen. A oligomers.1 The most thoroughly studied monomer commercial sample of EO was distilled twice. was ethylene oxide (EO). Eastham et a!. observed Tetrahydropyran (THP) and DON were dried with that the cationic polymerization of EO resulted in sodium metal and distilled. -

Uncovering the Energies and Reactivity of Aminoxyl Radicals by Mass Spectrometry David Marshall University of Wollongong
University of Wollongong Research Online University of Wollongong Thesis Collection University of Wollongong Thesis Collections 2014 Uncovering the Energies and Reactivity of Aminoxyl Radicals by Mass Spectrometry David Marshall University of Wollongong Research Online is the open access institutional repository for the University of Wollongong. For further information contact the UOW Library: [email protected] Uncovering the Energetics and Reactivity of Aminoxyl Radicals by Mass Spectrometry A thesis submitted in (partial) fulfilment of the requirements for the award of the Degree Doctor of Philosophy from University of Wollongong by David Lachlan Marshall BNanotechAdv (Hons I) School of Chemistry April 2014 DECLARATION I, David L. Marshall, declare that this thesis, submitted in fulfilment of the requirements for the award of Doctor of Philosophy, in the School of Chemistry, University of Wollongong, is wholly my own work unless otherwise referenced or acknowledged. The document has not been submitted for qualifications at any other academic institution. David L. Marshall April 2014 i ACKNOWLDGEMENTS This PhD would not have been possible without the support of many people, and I am strongly indebted to each and every one of them. First and foremost, a big thankyou to my supervisors Prof. Stephen Blanksby and Dr. Philip Barker. Over the past 7 years, through undergraduate research, Honours and into a PhD, you have been a constant source of ideas, encouragement, and support. Phil, your passion for science is contagious, and hopefully I’ve learned a few Ninja skills along the way. Leaving Wollongong won’t feel right without your face greeting me from the side of a truck every day. -

Reactions of Aromatic Compounds Just Like an Alkene, Benzene Has Clouds of Electrons Above and Below Its Sigma Bond Framework
Reactions of Aromatic Compounds Just like an alkene, benzene has clouds of electrons above and below its sigma bond framework. Although the electrons are in a stable aromatic system, they are still available for reaction with strong electrophiles. This generates a carbocation which is resonance stabilized (but not aromatic). This cation is called a sigma complex because the electrophile is joined to the benzene ring through a new sigma bond. The sigma complex (also called an arenium ion) is not aromatic since it contains an sp3 carbon (which disrupts the required loop of p orbitals). Ch17 Reactions of Aromatic Compounds (landscape).docx Page1 The loss of aromaticity required to form the sigma complex explains the highly endothermic nature of the first step. (That is why we require strong electrophiles for reaction). The sigma complex wishes to regain its aromaticity, and it may do so by either a reversal of the first step (i.e. regenerate the starting material) or by loss of the proton on the sp3 carbon (leading to a substitution product). When a reaction proceeds this way, it is electrophilic aromatic substitution. There are a wide variety of electrophiles that can be introduced into a benzene ring in this way, and so electrophilic aromatic substitution is a very important method for the synthesis of substituted aromatic compounds. Ch17 Reactions of Aromatic Compounds (landscape).docx Page2 Bromination of Benzene Bromination follows the same general mechanism for the electrophilic aromatic substitution (EAS). Bromine itself is not electrophilic enough to react with benzene. But the addition of a strong Lewis acid (electron pair acceptor), such as FeBr3, catalyses the reaction, and leads to the substitution product. -

1 050929 Quiz 1 Introduction to Polymers 1) in Class We Discussed
050929 Quiz 1 Introduction to Polymers 1) In class we discussed a hierarchical categorization of the terms: macromolecule, polymer and plastics, in that plastics are polymers and macromolecules and polymers are macromolecules; but all macromolecules are not polymers and all polymers are not plastics. a) Give an example of a macromolecule that is not a polymer. b) Give an example of a polymer that is not a plastic. c) Define polymer. d) Define plastic. e) Define macromolecule. 2) In class we observed a simulation of a small polymer chain of 40 flexible steps. The chain was observed to explore configurational space. a) Explain what the phrase "explore configurational space" means. b) Consider the two ends of the chain to be tethered with a separation distance of about 6 extended steps. If temperature were increased would the chain ends push apart or pull together the tether points? Explain why. c) If one chain end is fixed in Cartesian space at x = 0, what are the limits on the x-axis for the other chain end as it explores configurational space? d) What is the average value of the position on the x-axis? dγ 3) Polymer melts are processed at high rates of strain, , in an extruder, injection molder, or dt calander. a) Sketch a typical plot of polymer viscosity versus rate of strain on a log-log plot showing the power-law region and the Newtonian plateau at low rates of strain. b) Explain using this plot why polymers are processed at high rates of strain. c) Using your own words propose a molecular model that could explain shear thinning in polymers. -

Kinetics and Modeling of the Radical Polymerization of Acrylic Acid and of Methacrylic Acid in Aqueous Solution
Kinetics and Modeling of the Radical Polymerization of Acrylic Acid and of Methacrylic Acid in Aqueous Solution Dissertation zur Erlangung des mathematisch-naturwissenschaftlichen Doktorgrades „Doctor rerum naturalium“ der Georg-August-Universität Göttingen im Promotionsprogramm Chemie der Georg-August University School of Science (GAUSS) vorgelegt von Nils Friedrich Gunter Wittenberg aus Hamburg Göttingen, 2013 Betreuungsausschuss Prof. Dr. M. Buback, Technische und Makromolekulare Chemie, Institut für Physikalische Chemie, Georg-August-Universität Göttingen Prof. Dr. P. Vana, MBA, Makromolekulare Chemie, Institut für Physikalische Chemie, Georg-August-Universität Göttingen Mitglieder der Prüfungskommission Referent: Prof. Dr. M. Buback, Technische und Makromolekulare Chemie, Institut für Physikalische Chemie, Georg-August-Universität Göttingen Korreferent: Prof. Dr. P. Vana, MBA, Makromolekulare Chemie, Institut für Physikalische Chemie, Georg-August-Universität Göttingen Weitere Mitglieder der Prüfungskommission: Prof. Dr. G. Echold, Physikalische Chemie fester Körper, Institut für Physikalische Chemie, Georg-August-Universität Göttingen Prof. Dr. B. Geil, Biophysikalische Chemie, Institut für Physikalische Chemie, Georg-August- Universität Göttingen Jun.-Prof. Dr. R. Mata, Computerchemie und Biochemie,Institut für Physikalische Chemie, Georg-August-Universität Göttingen Prof. Dr. A. Wodtke, Physikalische Chemie I / Humboldt-Professur, Institut für Physikalische Chemie, Georg-August-Universität Göttingen Tag der mündlichen Prüfung: -

United States Patent (19) 11 Patent Number: 4,507,439 Stewart 45) Date of Patent: Mar
United States Patent (19) 11 Patent Number: 4,507,439 Stewart 45) Date of Patent: Mar. 26, 1985 54 ELASTOMERIC COMPOSITIONS CONTAINING TREATED PTFE OTHER PUBLICATIONS A. A. Benderly, Treatment of Teflon to Promote Bond 75) Inventor: Charles W. Stewart, Newark, Del. ability, J. Appl. Polymer Science VI, 20, 221-225, 73) Assignee: E. I. Du Pont de Nemours and Company, Wilmington, Del. (1962). Primary Examiner-Carman J. Seccuro 21 Appl. No.: 555,488 Attorney, Agent, or Firm-Paul R. Steyermark 22 Filed: Nov. 28, 1983 57 ABSTRACT 51 Int. Cl. ...................... C08L 27/22; C08L 27/16; An elastomeric composition comprising an elastomer C08L 27/18 matrix having dispersed therein about 3-30 parts per 52 U.S. Cl. .................................... 525/199; 525/200; 100 parts by weight of the elastomer of powdered poly 525/195; 525/196; 525/326.2, 525/366; tetrafluoroethylene having a number average molecular 525/367 weight of at least about 250,000 and a surface area of at 58 Field of Search ........................ 525/199, 195, 196 least 1 m2/g which has been treated with about 56) . References Cited 50-120% of sodium-naphthalene addition compound or U.S. PATENT DOCUMENTS another addition compound of an alkali metal required for the alkali metal to react with all the fluorine atoms 2,710,290 6/1955 Safford et al. ........................ 260/41 2,871,144 1/1959 Doban ........... ... 117/138.8 on the surface of the polytetrafluoroethylene powder. 3,019,206 1/1962 Robb ................................... 525/199 By dispersing such treated polytetrafluoroethylene 3,940,455 2/1976 Kaufman ............................ -

Living Free Radical Polymerization with Reversible Addition – Fragmentation
Polymer International Polym Int 49:993±1001 (2000) Living free radical polymerization with reversible addition – fragmentation chain transfer (the life of RAFT) Graeme Moad,* John Chiefari, (Bill) YK Chong, Julia Krstina, Roshan TA Mayadunne, Almar Postma, Ezio Rizzardo and San H Thang CSIRO Molecular Science, Bag 10, Clayton South 3169, Victoria, Australia Abstract: Free radical polymerization with reversible addition±fragmentation chain transfer (RAFT polymerization) is discussed with a view to answering the following questions: (a) How living is RAFT polymerization? (b) What controls the activity of thiocarbonylthio compounds in RAFT polymeriza- tion? (c) How do rates of polymerization differ from those of conventional radical polymerization? (d) Can RAFT agents be used in emulsion polymerization? Retardation, observed when high concentra- tions of certain RAFT agents are used and in the early stages of emulsion polymerization, and how to overcome it by appropriate choice of reaction conditions, are considered in detail. Examples of the use of thiocarbonylthio RAFT agents in emulsion and miniemulsion polymerization are provided. # 2000 Society of Chemical Industry Keywords: living polymerization; controlled polymerization; radical polymerization; dithioester; trithiocarbonate; transfer agent; RAFT; star; block; emulsion INTRODUCTION carbonylthio compounds 2 by addressing the following In recent years, considerable effort1,2 has been issues: expended to develop free radical processes that display (a) How living is RAFT polymerization? -

Analysis of Alternatives
ANALYSIS OF ALTERNATIVES Legal name of applicant(s): Acton Technologies Ltd Submitted by: Acton Technologies Ltd (jointly developed with Maflon Spa) Substance: bis(2-methoxyethyl) ether (diglyme): EC 203-924-4: CAS 111-96-6 Use title: Use of bis(2-methoxyethyl) ether (diglyme) as a carrier solvent in the formulation and use of sodium naphthalide etchant for fluoropolymer surface modification whilst preserving article structural integrity (in-house processes). Use of bis(2-methoxyethyl) ether (diglyme) as a carrier solvent in the application of sodium naphthalide etchant for fluoropolymer surface modification whilst preserving article structural integrity (downstream user processes). Use number: 1 and 2 ANALYSIS OF ALTERNATIVES CONTENTS LIST OF ABBREVIATIONS ....................................................................................................................................... 5 DECLARATION .......................................................................................................................................................... 6 1. SUMMARY ............................................................................................................................................................ 7 2. ANALYSIS OF SUBSTANCE FUNCTION.......................................................................................................... 10 2.1. The requirement to modify the surface of fluoropolymers ............................................................................. 10 2.1.1 Fluoropolymers .................................................................................................................................. -

Polymer Chemistry
Polymer Chemistry Properties and Application Bearbeitet von Andrew J Peacock, Allison Calhoun 1. Auflage 2006. Buch. XIX, 399 S. Hardcover ISBN 978 3 446 22283 0 Format (B x L): 17,5 x 24,5 cm Gewicht: 1088 g Weitere Fachgebiete > Chemie, Biowissenschaften, Agrarwissenschaften > Biochemie > Polymerchemie Zu Inhaltsverzeichnis schnell und portofrei erhältlich bei Die Online-Fachbuchhandlung beck-shop.de ist spezialisiert auf Fachbücher, insbesondere Recht, Steuern und Wirtschaft. Im Sortiment finden Sie alle Medien (Bücher, Zeitschriften, CDs, eBooks, etc.) aller Verlage. Ergänzt wird das Programm durch Services wie Neuerscheinungsdienst oder Zusammenstellungen von Büchern zu Sonderpreisen. Der Shop führt mehr als 8 Millionen Produkte. Produktinformation Seite 1 von 1 Polymer Chemistry Allison Calhoun, Andrew James Peacock Properties and Application ISBN 3-446-22283-9 Leseprobe Weitere Informationen oder Bestellungen unter http://www.hanser.de/3-446-22283-9 sowie im Buchhandel http://www.hanser.de/deckblatt/deckblatt1.asp?isbn=3-446-22283-9&style=Leseprobe 05.05.2006 2 Polymer Chemistry 2.1 Introduction In this chapter, we will see how polymers are manufactured from monomers. We will explore the chemical mechanisms that create polymers as well as how polymerization methods affect the final molecular structure of the polymer. We will look at the effect of the chemical structure of monomers, catalysts, radicals, and solvents on polymeric materials. Finally, we will apply our molecular understanding to the real world problem of producing polymers on a commercial scale. 2.2 Thermoplastics and Thermosets Thermoplastics consist of linear or lightly branched chains that can slide past one another under the influence of temperature and pressure.