Sable Antelope, Hippotragus Niger
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Hippotragus Equinus – Roan Antelope
Hippotragus equinus – Roan Antelope authorities as there may be no significant genetic differences between the two. Many of the Roan Antelope in South Africa are H. e. cottoni or equinus x cottoni (especially on private properties). Assessment Rationale This charismatic antelope exists at low density within the assessment region, occurring in savannah woodlands and grasslands. Currently (2013–2014), there are an observed 333 individuals (210–233 mature) existing on nine formally protected areas within the natural distribution range. Adding privately protected subpopulations and an Cliff & Suretha Dorse estimated 0.8–5% of individuals on wildlife ranches that may be considered wild and free-roaming, yields a total mature population of 218–294 individuals. Most private Regional Red List status (2016) Endangered subpopulations are intensively bred and/or kept in camps C2a(i)+D*†‡ to exclude predators and to facilitate healthcare. Field National Red List status (2004) Vulnerable D1 surveys are required to identify potentially eligible subpopulations that can be included in this assessment. Reasons for change Non-genuine: While there was an historical crash in Kruger National Park New information (KNP) of 90% between 1986 and 1993, the subpopulation Global Red List status (2008) Least Concern has since stabilised at c. 50 individuals. Overall, over the past three generations (1990–2015), based on available TOPS listing (NEMBA) Vulnerable data for nine formally protected areas, there has been a CITES listing None net population reduction of c. 23%, which indicates an ongoing decline but not as severe as the historical Endemic Edge of Range reduction. Further long-term data are needed to more *Watch-list Data †Watch-list Threat ‡Conservation Dependent accurately estimate the national population trend. -

Animals of Africa
Silver 49 Bronze 26 Gold 59 Copper 17 Animals of Africa _______________________________________________Diamond 80 PYGMY ANTELOPES Klipspringer Common oribi Haggard oribi Gold 59 Bronze 26 Silver 49 Copper 17 Bronze 26 Silver 49 Gold 61 Copper 17 Diamond 80 Diamond 80 Steenbok 1 234 5 _______________________________________________ _______________________________________________ Cape grysbok BIG CATS LECHWE, KOB, PUKU Sharpe grysbok African lion 1 2 2 2 Common lechwe Livingstone suni African leopard***** Kafue Flats lechwe East African suni African cheetah***** _______________________________________________ Red lechwe Royal antelope SMALL CATS & AFRICAN CIVET Black lechwe Bates pygmy antelope Serval Nile lechwe 1 1 2 2 4 _______________________________________________ Caracal 2 White-eared kob DIK-DIKS African wild cat Uganda kob Salt dik-dik African golden cat CentralAfrican kob Harar dik-dik 1 2 2 African civet _______________________________________________ Western kob (Buffon) Guenther dik-dik HYENAS Puku Kirk dik-dik Spotted hyena 1 1 1 _______________________________________________ Damara dik-dik REEDBUCKS & RHEBOK Brown hyena Phillips dik-dik Common reedbuck _______________________________________________ _______________________________________________African striped hyena Eastern bohor reedbuck BUSH DUIKERS THICK-SKINNED GAME Abyssinian bohor reedbuck Southern bush duiker _______________________________________________African elephant 1 1 1 Sudan bohor reedbuck Angolan bush duiker (closed) 1 122 2 Black rhinoceros** *** Nigerian -

Transboundary Species Project
TRANSBOUNDARY SPECIES PROJECT ROAN, SABLE AND TSESSEBE Rowan B. Martin Species Report for Roan, Sable and Tsessebe in support of The Transboundary Mammal Project of the Ministry of Environment and Tourism, Namibia facilitated by The Namibia Nature Foundation and World Wildlife Fund Living in a Finite Environment (LIFE) Programme Cover picture adapted from the illustrations by Clare Abbott in The Mammals of the Southern African Subregion by Reay H.N. Smithers Published by the University of Pretoria Republic of South Africa 1983 Transboundary Species Project – Background Study Roan, Sable and Tsessebe CONTENTS 1. BIOLOGICAL INFORMATION ...................................... 1 a. Taxonomy ..................................................... 1 b. Physical description .............................................. 3 c. Habitat ....................................................... 6 d. Reproduction and Population Dynamics ............................. 12 e. Distribution ................................................... 14 f. Numbers ..................................................... 24 g. Behaviour .................................................... 38 h. Limiting Factors ............................................... 40 2. SIGNIFICANCE OF THE THREE SPECIES ........................... 43 a. Conservation Significance ........................................ 43 b. Economic Significance ........................................... 44 3. STAKEHOLDING ................................................. 48 a. Stakeholders ................................................. -

Chapter 15 the Mammals of Angola
Chapter 15 The Mammals of Angola Pedro Beja, Pedro Vaz Pinto, Luís Veríssimo, Elena Bersacola, Ezequiel Fabiano, Jorge M. Palmeirim, Ara Monadjem, Pedro Monterroso, Magdalena S. Svensson, and Peter John Taylor Abstract Scientific investigations on the mammals of Angola started over 150 years ago, but information remains scarce and scattered, with only one recent published account. Here we provide a synthesis of the mammals of Angola based on a thorough survey of primary and grey literature, as well as recent unpublished records. We present a short history of mammal research, and provide brief information on each species known to occur in the country. Particular attention is given to endemic and near endemic species. We also provide a zoogeographic outline and information on the conservation of Angolan mammals. We found confirmed records for 291 native species, most of which from the orders Rodentia (85), Chiroptera (73), Carnivora (39), and Cetartiodactyla (33). There is a large number of endemic and near endemic species, most of which are rodents or bats. The large diversity of species is favoured by the wide P. Beja (*) CIBIO-InBIO, Centro de Investigação em Biodiversidade e Recursos Genéticos, Universidade do Porto, Vairão, Portugal CEABN-InBio, Centro de Ecologia Aplicada “Professor Baeta Neves”, Instituto Superior de Agronomia, Universidade de Lisboa, Lisboa, Portugal e-mail: [email protected] P. Vaz Pinto Fundação Kissama, Luanda, Angola CIBIO-InBIO, Centro de Investigação em Biodiversidade e Recursos Genéticos, Universidade do Porto, Campus de Vairão, Vairão, Portugal e-mail: [email protected] L. Veríssimo Fundação Kissama, Luanda, Angola e-mail: [email protected] E. -

Understanding the Behavioural Trade-Offs Made by Blue Wildebeest (Connochaetes Taurinus): the Importance of Resources, Predation and the Landscape
Understanding the behavioural trade-offs made by blue wildebeest (Connochaetes taurinus): the importance of resources, predation and the landscape Rebecca Dannock Bachelor of Science (Hons) in Zoology A thesis submitted for the degree of Doctor of Philosophy at The University of Queensland in 2016 School of Biological Sciences Abstract Prey individuals must constantly make decisions regarding safety and resource acquisition to ensure that they acquire enough resources without being predated upon. These decisions result in a trade-off between resource acquisition behaviours (such as foraging and drinking) and safety behaviours (such as grouping and vigilance). This trade- off is likely to be affected by the social and environmental factors that an individual experiences, including the individual’s location in the landscape. The overall objective of my PhD was to understand the decisions a migratory ungulate makes in order to acquire enough resources, while not becoming prey, and to understand how these decisions are influenced by social and environmental factors. In order to do this, I studied the behaviour of blue wildebeest (Connochaetes taurinus) in Etosha National Park, Namibia, between 2013 and 2015. I studied wildebeests’ behaviour while they acquired food and water and moved within the landscape. Along with observational studies, I also used lion (Panthera leo) roar playbacks to experimentally manipulate perceived predator presence to test wildebeests’ responses to immediate predation risk. For Chapter 2 I studied the foraging-vigilance trade-off of wildebeest to determine how social and environmental factors, including the location within the landscape, were correlated with wildebeests’ time spent foraging and vigilant as well as their bite rate. -

Sustainable Wildlife Management
249ISSN 0041-6436 An international journal of forestry and forest industries Vol. 68 2017/1 SUSTAINABLE WILDLIFE MANAGEMENT 249ISSN 0041-6436 An international journal of forestry and forest industries Vol. 68 2017/1 Editor: A. Sarre Editorial Advisory Board: S. Braatz, I. Buttoud, P. Csoka, L. Flejzor, T. Hofer, Contents F. Kafeero, W. Kollert, S. Lapstun, D. Mollicone, D. Reeb, S. Rose, J. Tissari, Editorial 2 P. van Lierop Emeritus Advisers: J. Ball, I.J. Bourke, R. Cooney, C. Freese, H. Dublin, D. Roe, D. Mallon, M. Knight, C. Palmberg-Lerche, L. Russo R. Emslie, M. Pani, V. Booth, S. Mahoney and C. Buyanaa Regional Advisers: F. Bojang, P. Durst, The baby and the bathwater: trophy hunting, conservation A.A. Hamid, J. Meza and rural livelihoods 3 Unasylva is published in English, French J. Stahl and T. De Meulenaer and Spanish. Subscriptions can be obtained CITES and the international trade in wildlife 17 by sending an e-mail to [email protected]. Subscription requests from institutions Y. Vizina and D. Kobei (e.g. libraries, companies, organizations, Indigenous peoples and sustainable wildlife management universities) rather than individuals are in the global era 27 preferred in order to make the journal accessible to more readers. D. Roe, R. Cooney, H. Dublin, D. Challender, D. Biggs, D. Skinner, All issues of Unasylva are available online M. Abensperg-Traun, N. Ahlers, R. Melisch and M. Murphree free of charge at www.fao.org/forestry/ First line of defence: engaging communities in tackling unasylva. Comments and queries are welcome: wildlife crime 33 [email protected]. -

Mixed-Species Exhibits with Pigs (Suidae)
Mixed-species exhibits with Pigs (Suidae) Written by KRISZTIÁN SVÁBIK Team Leader, Toni’s Zoo, Rothenburg, Luzern, Switzerland Email: [email protected] 9th May 2021 Cover photo © Krisztián Svábik Mixed-species exhibits with Pigs (Suidae) 1 CONTENTS INTRODUCTION ........................................................................................................... 3 Use of space and enclosure furnishings ................................................................... 3 Feeding ..................................................................................................................... 3 Breeding ................................................................................................................... 4 Choice of species and individuals ............................................................................ 4 List of mixed-species exhibits involving Suids ........................................................ 5 LIST OF SPECIES COMBINATIONS – SUIDAE .......................................................... 6 Sulawesi Babirusa, Babyrousa celebensis ...............................................................7 Common Warthog, Phacochoerus africanus ......................................................... 8 Giant Forest Hog, Hylochoerus meinertzhageni ..................................................10 Bushpig, Potamochoerus larvatus ........................................................................ 11 Red River Hog, Potamochoerus porcus ............................................................... -

List of 28 Orders, 129 Families, 598 Genera and 1121 Species in Mammal Images Library 31 December 2013
What the American Society of Mammalogists has in the images library LIST OF 28 ORDERS, 129 FAMILIES, 598 GENERA AND 1121 SPECIES IN MAMMAL IMAGES LIBRARY 31 DECEMBER 2013 AFROSORICIDA (5 genera, 5 species) – golden moles and tenrecs CHRYSOCHLORIDAE - golden moles Chrysospalax villosus - Rough-haired Golden Mole TENRECIDAE - tenrecs 1. Echinops telfairi - Lesser Hedgehog Tenrec 2. Hemicentetes semispinosus – Lowland Streaked Tenrec 3. Microgale dobsoni - Dobson’s Shrew Tenrec 4. Tenrec ecaudatus – Tailless Tenrec ARTIODACTYLA (83 genera, 142 species) – paraxonic (mostly even-toed) ungulates ANTILOCAPRIDAE - pronghorns Antilocapra americana - Pronghorn BOVIDAE (46 genera) - cattle, sheep, goats, and antelopes 1. Addax nasomaculatus - Addax 2. Aepyceros melampus - Impala 3. Alcelaphus buselaphus - Hartebeest 4. Alcelaphus caama – Red Hartebeest 5. Ammotragus lervia - Barbary Sheep 6. Antidorcas marsupialis - Springbok 7. Antilope cervicapra – Blackbuck 8. Beatragus hunter – Hunter’s Hartebeest 9. Bison bison - American Bison 10. Bison bonasus - European Bison 11. Bos frontalis - Gaur 12. Bos javanicus - Banteng 13. Bos taurus -Auroch 14. Boselaphus tragocamelus - Nilgai 15. Bubalus bubalis - Water Buffalo 16. Bubalus depressicornis - Anoa 17. Bubalus quarlesi - Mountain Anoa 18. Budorcas taxicolor - Takin 19. Capra caucasica - Tur 20. Capra falconeri - Markhor 21. Capra hircus - Goat 22. Capra nubiana – Nubian Ibex 23. Capra pyrenaica – Spanish Ibex 24. Capricornis crispus – Japanese Serow 25. Cephalophus jentinki - Jentink's Duiker 26. Cephalophus natalensis – Red Duiker 1 What the American Society of Mammalogists has in the images library 27. Cephalophus niger – Black Duiker 28. Cephalophus rufilatus – Red-flanked Duiker 29. Cephalophus silvicultor - Yellow-backed Duiker 30. Cephalophus zebra - Zebra Duiker 31. Connochaetes gnou - Black Wildebeest 32. Connochaetes taurinus - Blue Wildebeest 33. Damaliscus korrigum – Topi 34. -
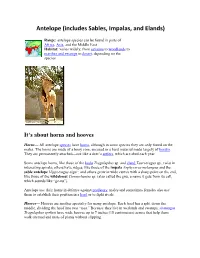
Antelope (Includes Sables, Impalas, and Elands)
Antelope (includes Sables, Impalas, and Elands) Range: antelope species can be found in parts of Africa, Asia, and the Middle East Habitat: varies widely, from savanna to woodlands to marshes and swamps to desert, depending on the species It’s about horns and hooves Horns— All antelope species have horns, although in some species they are only found on the males. The horns are made of a bony core, encased in a hard material made largely of keratin. They are permanently attached—not like a deer’s antlers, which are shed each year. Some antelope horns, like those of the kudu Tragelaphus sp. and eland Taurotragus sp., twist in interesting spirals; others have ridges, like those of the impala Aephyceros melampus and the sable antelope Hippotragus niger; and others grow in wide curves with a sharp point on the end, like those of the wildebeest Connochaetes sp. (also called the gnu, a name it gets from its call, which sounds like “ge-nu”). Antelope use their horns in defense against predators; males and sometimes females also use them to establish their position in a herd or to fight rivals Hooves— Hooves are another specialty for many antelope. Each hoof has a split down the middle, dividing the hoof into two “toes.” Because they live in wetlands and swamps, sitatungas Tragelaphus spekeii have wide hooves up to 7 inches (18 centimeters) across that help them walk on mud and mats of plants without slipping. Nile lechwes Kobus magaceros, which also live in swampy areas, have long, pointed hooves to give them sure footing in the water. -

Ecology and Conservation of Mini-Antelope: Proceedings of an International Symposium and on Duiker and Dwarf Antelope in Africa
SPECIES CONCEPTS AND THE REAL DIVERSITY OF ANTELOPES F. P. D. COTTERILL Principal Curator of Vertebrates, Department of Mammalogy, Natural History Museum of Zimbabwe, P. O. Box 240, Bulawayo, Zimbabwe 2003. In A. Plowman (Ed.). Ecology and Conservation of Mini-antelope: Proceedings of an International Symposium and on Duiker and Dwarf Antelope in Africa. Filander Verlag: Füürth. pp. 59-118. Biodiversity Foundation for Africa, Secretariat: P O Box FM730, Famona, Bulawayo, Zimbabwe (Address for correspondence) email: [email protected] Abstract As for all biodiversity, society requires an accurate taxonomy of the Bovidae. We need to know what the different antelopes really are, and where these occur. Sound scientific, economic and aesthetic arguments underpin this rationale. This paper highlights some of the costs that result from the misconstrual of the real nature of species. Controversy reigns over which species concepts are most applicable to characterize biodiversity; a controversy magnified by how different species concepts create taxonomies of differing accuracy and precision. The contemporary taxonomy of the Mammalia continues to be based on the Biological Species Concept (BSC). Its deficiencies are too rarely acknowledged, and afflict apparently well known taxa of large mammals, notably the Bovidae. Errors in the BSC misconstrue natural patterns of diversity: recognizing too many (Type I errors), or too few species (Type II errors). Most insidious are Type III errors; where evolutionary relationships are misconstrued because the BSC cannot conceptualize, and thus ignores phylogenetic uniqueness. The general trend in current taxonomies of antelopes is to under represent true diversity - exemplified in the dikdiks (Type II errors). Misconstrual of phylogenetic relationships among species (Type III errors) appear rampant in these same taxonomies (the Cephalophini for example). -

GNUSLETTER Vol 37#2.Pdf
GNUSLETTER Volume 37 / Number 2 December 2020 ANTELOPE SPECIALIST GROUP IUCN Species Survival Commission Antelope Specialist Group GNUSLETTER is the biannual newsletter of the IUCN Species Survival Commission Antelope Specialist Group (ASG). First published in 1982 by first ASG Chair Richard D. Estes, the intent of GNUSLETTER, then and today, is the dissemination of reports and information regarding antelopes and their conservation. ASG Members are an important network of individuals and experts working across disciplines throughout Africa, Asia and America. Contributions (original articles, field notes, other material relevant to antelope biology, ecology, and conservation) are welcomed and should be sent to the editor. Today GNUSLETTER is published in English in electronic form and distributed widely to members and non-members, and to the IUCN SSC global conservation network. To be added to the distribution list please contact [email protected]. GNUSLETTER Editorial Board - David Mallon, ASG Co-Chair - Philippe Chardonnet, ASG Co-Chair ASG Program Office - Tania Gilbert, Marwell Wildife The Antelope Specialist Group Program Office is hosted and supported by Marwell Wildlife https://www.marwell.org.uk The designation of geographical entities in this report does not imply the expression of any opinion on the part of IUCN, the Species Survival Commission, or the Antelope Specialist Group concerning the legal status of any country, territory or area, or concerning the delimitation of any frontiers or boundaries. Views expressed in GNUSLETTER are those of the individual authors, Cover photo: Young female bushbuck (Tragelaphus scriptus), W National Park and Biosphere Reserve, Niger (© Daniel Cornélis) 2 GNUSLETTER Volume 37 Number 2 December 2020 FROM IUCN AND ASG………………………………………………………. -

Antelope (Includes Sables, Impalas, and Elands)
Antelope (includes Sables, Impalas, and Elands) Range: antelope species can be found in parts of Africa, Asia, and the Middle East Habitat: varies widely, from savanna to woodlands to marshes and swamps to desert, depending on the species It’s about horns and hooves Horns— All antelope species have horns, although in some species they are only found on the males. The horns are made of a bony core, encased in a hard material made largely of keratin. They are permanently attached—not like a deer’s antlers, which are shed each year. Some antelope horns, like those of the kudu Tragelaphus sp. and eland Taurotragus sp., twist in interesting spirals; others have ridges, like those of the impala Aephyceros melampus and the sable antelope Hippotragus niger; and others grow in wide curves with a sharp point on the end, like those of the wildebeest Connochaetes sp. (also called the gnu, a name it gets from its call, which sounds like “ge-nu”). Antelope use their horns in defense against predators; males and sometimes females also use them to establish their position in a herd or to fight rivals Hooves— Hooves are another specialty for many antelope. Each hoof has a split down the middle, dividing the hoof into two “toes.” Because they live in wetlands and swamps, sitatungas Tragelaphus spekeii have wide hooves up to 7 inches (18 centimeters) across that help them walk on mud and mats of plants without slipping. Nile lechwes Kobus magaceros, which also live in swampy areas, have long, pointed hooves to give them sure footing in the water.