The Only Erethizontidae with a Tenden
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Redalyc.A Distinctive New Cloud-Forest Rodent (Hystriocognathi: Echimyidae) from the Manu Biosphere Reserve, Peru
Mastozoología Neotropical ISSN: 0327-9383 [email protected] Sociedad Argentina para el Estudio de los Mamíferos Argentina Patterson, Bruce D.; Velazco, Paul M. A distinctive new cloud-forest rodent (Hystriocognathi: Echimyidae) from the Manu Biosphere Reserve, Peru Mastozoología Neotropical, vol. 13, núm. 2, julio-diciembre, 2006, pp. 175-191 Sociedad Argentina para el Estudio de los Mamíferos Tucumán, Argentina Available in: http://www.redalyc.org/articulo.oa?id=45713202 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Mastozoología Neotropical, 13(2):175-191, Mendoza, 2006 ISSN 0327-9383 ©SAREM, 2006 Versión on-line ISSN 1666-0536 www.cricyt.edu.ar/mn.htm A DISTINCTIVE NEW CLOUD-FOREST RODENT (HYSTRICOGNATHI: ECHIMYIDAE) FROM THE MANU BIOSPHERE RESERVE, PERU Bruce D. Patterson1 and Paul M. Velazco1, 2 1 Department of Zoology, Field Museum of Natural History, 1400 S. Lake Shore Dr, Chicago IL 60605-2496 USA. 2 Department of Biological Sciences, University of Illinois at Chicago, 845 W. Taylor St, Chicago IL 60607 USA ABSTRACT: Recent surveys in Peru’s Manu National Park and Biosphere Reserve uncovered a new species of hystricognath rodent, a spiny rat (Echimyidae) with dense, soft fur. Inhabiting Andean cloud-forests at 1900 m, the new rodent belongs to a radiation of “brush- tailed tree rats” previously known only from the Amazon, Orinoco, and other lowland river drainages. Phylogenetic analysis of morphology (cranial and dental characters) unambiguously allies the new species with species of Isothrix. -

Hystrix Africaeaustralis)
Reproduction in captive female Cape porcupines (Hystrix africaeaustralis) R. J. van Aarde Mammal Research Institute, University ofPretoria, Pretoria 0002, South Africa Summary. Captive females attained sexual maturity at an age of 9\p=n-\16months and con- ceived for the first time when 10\p=n-\25months old. Adult females were polyoestrous but did not cycle while lactating or when isolated from males. The length of the cycle varied from 17 to 42 days (mean \m=+-\s.d. 31\m=.\2\m=+-\6\m=.\5days; n = 43) and females experienced 3\p=n-\7 sterile cycles before conceiving. Pregnancy lasted for 93\p=n-\94days (93\m=.\5\m=+-\0\m=.\6days; N = 4) and litter intervals varied from 296 to 500 days (385 \m=+-\60\m=.\4;n = 10). Litter size varied from 1 to 3 (1\m=.\5\m=+-\0\m=.\66;n = 165) and the well-developed precocial young weighed 300\p=n-\400g (351 \m=+-\47\m=.\4g; n= 19) at birth. Captive females reproduced throughout the year with most litters (78\m=.\7%;n = 165) being produced between August and March. Introduction Cape porcupines (Hystrix africaeaustralis) inhabit tropical forests, woodlands, grassland savannas, semi-arid and arid environments throughout southern Africa. Despite this widespread distribution little attention has been given to these nocturnal, Old World hystricomorph rodents, which shelter and breed in subterranean burrows, rock crevices and caves. Some information on reproduction in female porcupines has been published on the crested porcupine (H. cristata) (Weir, 1967), the Himalayan porcupine (H. hodgsoni) (Gosling, 1980) and the Indian porcupine (H. -

Porcupine Wildlife Note
24. Porcupine The porcupine is a blackish, quill-armored, slow-moving rodent with an appetite for tree bark and salt. It lives in forests and often can be seen hunched into what appears to be a black ball high in a tree. While it does not occur in all parts of Pennsylvania, the porcupine is one of Pennsylvania’s best-known and most easily identified wild animals. Its taxonomic name is Erethizon dorsatum. The word “porcupine” comes from two Latin words, porcus (“swine”) and spina (“thorn”), which also reflects the species’ colloquial name, quill pig. In the East, porcupines inhabit Canada and New England south into Pennsylvania; they range through the northern Midwest and the Pacific Northwest, south in the forested Rocky Mountains nearly to Mexico, and north to Alaska. They live at all elevations from sea level to timberline. Biology Adult porcupines are about 30 inches in length, including a 6- to 10-inch tail. They weigh 9 to 15 pounds, with bigger, older adults weighing up to 20 pounds. Males are larger than females. The porcupine is North America’s second largest rodent; only the beaver is bigger. Porcupines have four incisors—two above and two below that are bright orange, strong and adapted to gnawing. Short-legged and stout, a porcupine has a pronounced arch to its back. Its skull is heavily constructed; the small, longest), yellow or white tipped with black, and lined with rounded head has a blunt muzzle, ears almost hidden in a foam-like material composed of many tiny air cells. An fur, and dull black eyes. -

Michael O. Woodburne1,* Alberto L. Cione2,**, and Eduardo P. Tonni2,***
Woodburne, M.O.; Cione, A.L.; and Tonni, E.P., 2006, Central American provincialism and the 73 Great American Biotic Interchange, in Carranza-Castañeda, Óscar, and Lindsay, E.H., eds., Ad- vances in late Tertiary vertebrate paleontology in Mexico and the Great American Biotic In- terchange: Universidad Nacional Autónoma de México, Instituto de Geología and Centro de Geociencias, Publicación Especial 4, p. 73–101. CENTRAL AMERICAN PROVINCIALISM AND THE GREAT AMERICAN BIOTIC INTERCHANGE Michael O. Woodburne1,* Alberto L. Cione2,**, and Eduardo P. Tonni2,*** ABSTRACT The age and phyletic context of mammals that dispersed between North and South America during the past 9 m.y. is summarized. The presence of a Central American province of cladogenesis and faunal differentiation is explored. One apparent aspect of such a province is to delay dispersals of some taxa northward from Mexico into the continental United States, largely during the Blancan. Examples are recognized among the various xenar- thrans, and cervid artiodactyls. Whereas the concept of a Central American province has been mentioned in past investigations it is upgraded here. Paratoceras (protoceratid artio- dactyl) and rhynchotheriine proboscideans provide perhaps the most compelling examples of Central American cladogenesis (late Arikareean to early Barstovian and Hemphillian to Rancholabrean, respectively), but this category includes Hemphillian sigmodontine rodents, and perhaps a variety of carnivores and ungulates from Honduras in the medial Miocene, as well as peccaries and equids from Mexico. For South America, Mexican canids and hy- drochoerid rodents may have had an earlier development in Mexico. Remarkably, the first South American immigrants to Mexico (after the Miocene heralds; the xenarthrans Plaina and Glossotherium) apparently dispersed northward at the same time as the first Holarctic taxa dispersed to South America (sigmodontine rodents and the tayassuid artiodactyls). -

Dental Homologies and Evolutionary Transformations In
Dental homologies and evolutionary transformations in Caviomorpha (Hystricognathi, Rodentia): new data from the Paleogene of Peruvian Amazonia Myriam Boivin, Laurent Marivaux To cite this version: Myriam Boivin, Laurent Marivaux. Dental homologies and evolutionary transformations in Caviomor- pha (Hystricognathi, Rodentia): new data from the Paleogene of Peruvian Amazonia. Historical Biology, Taylor & Francis, 2020, 32 (4), pp.528-554. 10.1080/08912963.2018.1506778. hal-01870927 HAL Id: hal-01870927 https://hal.umontpellier.fr/hal-01870927 Submitted on 17 Jun 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Page 1 of 118 Historical Biology 1 2 3 Dental homologies and evolutionary transformations in Caviomorpha (Hystricognathi, 4 5 Rodentia): new data from the Paleogene of Peruvian Amazonia 6 7 8 9 10 a* a 11 Myriam Boivin and Laurent Marivaux 12 13 14 15 a Laboratoire de Paléontologie, Institut des Sciences de l’Évolution de Montpellier (ISE-M), c.c. 16 For Peer Review Only 17 18 064, Université de Montpellier, CNRS, IRD, EPHE, place Eugène Bataillon, F-34095 19 20 Montpellier Cedex 05, France. 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 *Corresponding author. -

Porcupine, Tree
UWI The Online Guide to the Animals of Trinidad and Tobago Behaviour Coendou prehensilis (Tree Porcupine or Brazilian Porcupine) Family: Erethizontidae (New World Porcupines) Order: Rodentia (Rodents) Class: Mammalia (Mammals) Fig. 1. Brazilian porcupine, Coendou prehensilis. [http://animaldiversity.ummz.umich.edu/site/resources/pablo_goncalves/Coendou2a.jpg/view.html, downloaded 22 November 2011] TRAITS. Coendou prehensilis, the Brazilian porcupine, is a mid-sized rodent with evolutionarily modified body hair. This modified hair exists as keratin-toughened, needle-like, semi-hollow quills or spines that grow to approximately 6.5cm in length. Like all New World porcupines, spines grow singly out of the skin and possess minute barbs at the end of the shaft. These spines cover the entire body, except for its fleshy nose, its belly and a large portion of its prehensile tail, and are usually light in colour (white to burnished yellow); there are also darker brown to black (soft/ unmodified) hairs interspersed along the body. It has small round eyes; and, a rounded yet flattened snout. The snout is covered with very short and very fine hairs and has several long whiskers. The porcupine has two long incisors and the front of its mouth that grows continuously during life and lacks canine teeth. At adult weight, this porcupine can range from 2kg to 5kg (Roberts et al., 1984). The average length of an adult Brazilian porcupine is approximately 90cm with its tail contributing about half of that length. Coendou also has modified padded feet with four long clawed toes. At birth, the infants are approximately 50cm head to tail, 0.415kg, are covered in reddish brown hair and have soft natal quills about 1.5cm in length that harden a few days after it is born. -

Out of Europe: Investigating Hystrix Cristata (Rodentia: Hystricidae) Skull Morphometric Geographic Variability in Africa
Biogeographia – The Journal of Integrative Biogeography 36 (2021): a001 https://doi.org/10.21426/B636051379 Out of Europe: Investigating Hystrix cristata (Rodentia: Hystricidae) skull morphometric geographic variability in Africa FRANCESCO M. ANGELICI1*, PAOLO COLANGELO2, SPARTACO GIPPOLITI3 1 FIZV, Via Marco Aurelio 2, I-00184 Rome (Italy) 2 Consiglio Nazionale delle Ricerche, Istituto di Ricerca sugli Ecosistemi Terrestri, CNR-IRET, Via Salaria km 29.300, I-00015 Montelibretti, Rome (Italy) 3 Società Italiana per la Storia della Fauna ‘Giuseppe Altobello’, Viale Liegi 48, I-00198 Rome (Italy) * corresponding author: [email protected] Keywords: Crested porcupine, geographic variability, Hystrix cristata senegalica, Hystrix cristata galeata, North-East Africa, taxonomy. SUMMARY The crested porcupine Hystrix cristata is one of the most well-known members of the Family Hystricidae, yet very little is known regarding its geographic variability in Africa. Two alternative hypotheses exist; pre-1940s classical taxonomy supported the existence of a distinct Eastern African species, Hystrix galeata, whereas recent molecular data seem to support only a North-South separation inside one single species, with the geographic-ecological barrier represented by the Sahara desert. Our morphometric data support the recognition of Hystrix cristata senegalica Cuvier, 1822 as the sub- Saharan representative of the species with a clear morphological difference between the North African and sub-Saharan crested porcupines, which seem re-conductible mostly to size difference. Within H. c. senegalica, our analysis seems to support a weak separation between the West African and the East African samples. Owing to considerable qualitative skull differences and overlooked molecular data, the taxonomic status of H. galeata remains uncertain as well as the status of porcupines of North-East Africa (Nubia). -

Multiple Molecular Evidences for a Living Mammalian Fossil
Multiple molecular evidences for a living mammalian fossil Dorothe´ e Huchon†‡, Pascale Chevret§¶, Ursula Jordanʈ, C. William Kilpatrick††, Vincent Ranwez§, Paulina D. Jenkins‡‡, Ju¨ rgen Brosiusʈ, and Ju¨ rgen Schmitz‡ʈ †Department of Zoology, George S. Wise Faculty of Life Sciences, Tel Aviv University, Tel Aviv 69978, Israel; §Department of Paleontology, Phylogeny, and Paleobiology, Institut des Sciences de l’Evolution, cc064, Universite´Montpellier II, Place E. Bataillon, 34095 Montpellier Cedex 5, France; ʈInstitute of Experimental Pathology, University of Mu¨nster, D-48149 Mu¨nster, Germany; ††Department of Biology, University of Vermont, Burlington, VT 05405-0086; and ‡‡Department of Zoology, The Natural History Museum, London SW7 5BD, United Kingdom Edited by Francisco J. Ayala, University of California, Irvine, CA, and approved March 18, 2007 (received for review February 11, 2007) Laonastes aenigmamus is an enigmatic rodent first described in their classification as a diatomyid suggests that Laonastes is a 2005. Molecular and morphological data suggested that it is the living fossil and a ‘‘Lazarus taxon.’’ sole representative of a new mammalian family, the Laonastidae, The two research teams also disagreed on the taxonomic and a member of the Hystricognathi. However, the validity of this position of Laonastes. According to Jenkins et al. (2), Laonastes family is controversial because fossil-based phylogenetic analyses is either the most basal group of the hystricognaths (Fig. 2A)or suggest that Laonastes is a surviving member of the Diatomyidae, nested within the hystricognaths (Fig. 2B). According to Dawson a family considered to have been extinct for 11 million years. et al. (3), Laonastes and the other Diatomyidae are the sister According to these data, Laonastes and Diatomyidae are the sister clade of the family Ctenodactylidae (i.e., gundies), a family that clade of extant Ctenodactylidae (i.e., gundies) and do not belong does not belong to the Hystricognathi, but to which it is to the Hystricognathi. -

Rodentia: Erethizontidae) Therya, Vol
Therya E-ISSN: 2007-3364 [email protected] Asociación Mexicana de Mastozoología México LEON-ALVARADO, OMAR DANIEL; RAMÍREZ-CHAVES, HÉCTOR E. Morphological description of the glans penis and baculum of Coendou quichua (Rodentia: Erethizontidae) Therya, vol. 8, núm. 3, 2017, pp. 263-267 Asociación Mexicana de Mastozoología Baja California Sur, México Available in: http://www.redalyc.org/articulo.oa?id=402352772011 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative THERYA, 2017, Vol. 8 (3): 263-268 DOI: 10.12933/therya- 17-495 ISSN 2007-3364 Morphological description of the glans penis and baculum of Coendou quichua (Rodentia: Erethizontidae) OMAR D ANIEL LEON-ALVARA DO 1* AN D H ÉCT OR E. RAMÍREZ -CHAVES 2 1 Laboratorio de Sistemática y Biogeografía, Escuela de Biología, Facultad de Ciencias, Universidad Industrial de Santander. Carrera 27 9, A. A. 678, Bucaramanga, Colombia. E-mail: [email protected] (ODLA) 2 Departamento de Ciencias Biológicas, Facultad de Ciencias Exactas y Naturales, Universidad de Caldas. Calle 65 26-10, A. A. 275, Manizales, Colombia. E-mail: [email protected] * Corresponding author External morphology of the glans and baculum are important characters for specic delimitation, especially for rodents (Simson et al. 1995). However, for Erethizontidae there are few descriptive works; in fact, for Neotropical porcupines of the genus Coendou there is just one brief contribution for an indeterminate species by Pocock in 1922. -

New Radiometric 40Ar–39Ar Dates and Faunistic Analyses Refine
www.nature.com/scientificreports OPEN New radiometric 40Ar–39Ar dates and faunistic analyses refne evolutionary dynamics of Neogene vertebrate assemblages in southern South America Francisco J. Prevosti1,2*, Cristo O. Romano2,3, Analía M. Forasiepi2,3, Sidney Hemming4, Ricardo Bonini2,5, Adriana M. Candela2,6, Esperanza Cerdeño2,3, M. Carolina Madozzo Jaén2,7,8, Pablo E. Ortiz2,7, François Pujos2,3, Luciano Rasia2,6, Gabriela I. Schmidt2,9, Matias Taglioretti10,11,12, Ross D. E. MacPhee13 & Ulyses F. J. Pardiñas2,14,15 The vertebrate fossil record of the Pampean Region of Argentina occupies an important place in South American vertebrate paleontology. An abundance of localities has long been the main basis for constructing the chronostratigraphical/geochronological scale for the late Neogene–Quaternary of South America, as well as for understanding major patterns of vertebrate evolution, including the Great American Biotic Interchange. However, few independently-derived dates are available for constraining this record. In this contribution, we present new 40Ar/39Ar dates on escorias (likely the product of meteoric impacts) from the Argentinean Atlantic coast and statistically-based biochronological analyses that help to calibrate Late Miocene–Pliocene Pampean faunal successions. For the type areas of the Montehermosan and Chapadmalalan Ages/Stages, our results delimit their age ranges to 4.7–3.7 Ma and ca. 3.74–3.04 Ma, respectively. Additionally, from Buenos Aires Province, dates of 5.17 Ma and 4.33 Ma were recovered for “Huayquerian” and Montehermosan faunas. This information helps to better calibrate important frst appearances of allochthonous taxa in South America, including one of the oldest records for procyonids (7.24–5.95 Ma), cricetids (6.95– 5.46 Ma), and tayassuids (> 3.74 Ma, oldest high-confdence record). -

In the Pleistocene of South America: Biogeographic and Paleoenvironmental Implications
Journal of South American Earth Sciences 82 (2018) 76e90 Contents lists available at ScienceDirect Journal of South American Earth Sciences journal homepage: www.elsevier.com/locate/jsames The southernmost record of a large erethizontid rodent (Hystricomorpha: Erethizontoidea) in the Pleistocene of South America: Biogeographic and paleoenvironmental implications * Raúl I. Vezzosi a, , Leonardo Kerber b a Laboratorio de Paleontología de Vertebrados, Centro de Investigaciones Científicas y Transferencia de Tecnología a la Produccion, Consejo Nacional de Investigaciones Científicas y Tecnicas, Materi y Espana,~ E3105BWA, Diamante, Argentina b CAPPA - Centro de Apoio a Pesquisa Paleontologica da Quarta Colonia,^ Universidade Federal de Santa Maria, Sao~ Joao~ do Pol^esine, Rua Maximiliano Vizzotto, 598, CEP 97230-000, Brazil article info abstract Article history: The South American porcupines (Erethizontidae) are included in two genera: Chaetomys and Coendou. Received 19 October 2017 The latter is a very speciose taxon, with about 13 living species. During at least the late Plioceneeearly Received in revised form Pleistocene, erethizontids immigrated to Central and North America during the Great American Biotic 23 December 2017 Interchange. Although some Pleistocene fossils have been reported, the Quaternary history of this clade Accepted 24 December 2017 is still understudied. The only known extinct species is Coendou magnus. In this work, a fossil of a Available online 30 December 2017 porcupine is reported from an Upper Pleistocene fluvial sedimentary sequence cropping out in the Northern Pampa geomorphological region, Santa Fe Province, Argentina. Despite this group having Keywords: Fossil record different living forms widely distributed in South American Neotropical woodland habitats, the Pleis- Quaternary tocene occurrences of Erethizontidae are scarce and limited to Upper Pleistocene deposits from Bolivia, Regional extinction Brazil, and Uruguay. -
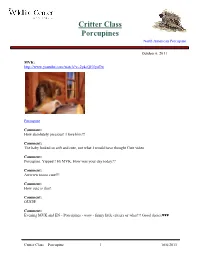
Critter Class Porcupines
Critter Class Porcupines North American Porcupine October 6, 2011 MVK: http://www.youtube.com/watch?v=2y4cQEEyuTw Porcupine Comment: How absolutely precious! I love him!!! Comment: The baby looked so soft and cute, not what I would have thought Cute video Comment: Porcupine. Yippee!! Hi MVK. How was your day today?? Comment: Awwww toooo cute!!! Comment: How cute is that! Comment: OUCH! Comment: Evening MVK and EN - Porcupines - wow - funny little critters or what!!! Good choice♥♥♥ Critter Class – Porcupine 1 10/6/2011 Comment: Oh my goodness -----how cute is that baby porcupine!! I really don't know much about them. He was using his paws so well. Do they have thumbs like raccoons? Comment: Oh my word, isn't that baby cute! And such a dainty eater! Comment: Is that a porcupine? Pulled many of quills from my dogs. Comment: Oh, one of my favorite animals. I love our type, and those snazzy African ones whose head look like they are wearing a fancy Parisian hat. I just love porcupines! MVK: Porcupines have soft hair, but on their back, sides, and tail it is usually mixed with sharp quills. These quills typically lie flat until a porcupine is threatened, then leap to attention as a persuasive deterrent. Porcupines cannot shoot them at predators as once thought, but the quills do detach easily when touched. from National Geographic MVK: http://www.youtube.com/watch?v=wYC0IYuOYLw&NR=1 MVK: http://www.youtube.com/watch?v=mqc5jJQwVu8&feature=relmfu MVK: This little porcupine's mother was hit and killed by a car.