In Vitro Selection and Evolution of Functional Proteins by Using Ribosome Display
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nilsson C. L. (Ed.)
Else_AT-NILSSON_prelims.qxd 6/8/2007 06:29 PM Page i Lectins Analytical Technologies This page intentionally left blank Else_AT-NILSSON_prelims.qxd 6/8/2007 06:29 PM Page iii Lectins Analytical Technologies Edited by Carol L. Nilsson National High Magnetic Field Laboratory Florida State University Tallahassee, FL, USA Amsterdam – Boston – Heidelberg – London – New York – Oxford – Paris San Diego – San Francisco – Singapore – Sydney – Tokyo Else_AT-NILSSON_prelims.qxd 6/8/2007 06:29 PM Page iv Elsevier Radarweg 29, PO Box 211, 1000 AE Amsterdam, The Netherlands Linacre House, Jordan Hill, Oxford OX2 8DP, UK First edition 2007 Copyright © 2007 Elsevier B.V. All rights reserved No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means electronic, mechanical, photocopying, recording or otherwise without the prior written permission of the publisher Permissions may be sought directly from Elsevier’s Science & Technology Rights Department in Oxford, UK: phone (+44) (0) 1865 843830; fax (+44) (0) 1865 853333; email: [email protected]. Alternatively you can submit your request online by visiting the Elsevier web site at http://www.elsevier.com/locate/permissions, and selecting Obtaining permission to use Elsevier material Notice No responsibility is assumed by the publisher for any injury and/or damage to persons or property as a matter of products liability, negligence or otherwise, or from any use or operation of any methods, products, instructions or ideas contained in the -
![M.Sc. [Botany] 346 13](https://docslib.b-cdn.net/cover/3507/m-sc-botany-346-13-923507.webp)
M.Sc. [Botany] 346 13
cover page as mentioned below: below: mentioned Youas arepage instructedcover the to updateupdate to the coverinstructed pageare asYou mentioned below: Increase the font size of the Course Name. Name. 1. IncreaseCourse the theof fontsize sizefont ofthe the CourseIncrease 1. Name. use the following as a header in the Cover Page. Page. Cover 2. the usein the followingheader a as as a headerfollowing the inuse the 2. Cover Page. ALAGAPPAUNIVERSITY UNIVERSITYALAGAPPA [Accredited with ’A+’ Grade by NAAC (CGPA:3.64) in the Third Cycle Cycle Third the in (CGPA:3.64) [AccreditedNAAC by withGrade ’A+’’A+’ Gradewith by NAAC[Accredited (CGPA:3.64) in the Third Cycle and Graded as Category–I University by MHRD-UGC] MHRD-UGC] by University and Category–I Graded as as Graded Category–I and University by MHRD-UGC] M.Sc. [Botany] 003 630 – KARAIKUDIKARAIKUDI – 630 003 346 13 EDUCATION DIRECTORATEDISTANCE OF OF DISTANCEDIRECTORATE EDUCATION BIOLOGICAL TECHNIQUES IN BOTANY I - Semester BOTANY IN TECHNIQUES BIOLOGICAL M.Sc. [Botany] 346 13 cover page as mentioned below: below: mentioned Youas arepage instructedcover the to updateupdate to the coverinstructed pageare asYou mentioned below: Increase the font size of the Course Name. Name. 1. IncreaseCourse the theof fontsize sizefont ofthe the CourseIncrease 1. Name. use the following as a header in the Cover Page. Page. Cover 2. the usein the followingheader a as as a headerfollowing the inuse the 2. Cover Page. ALAGAPPAUNIVERSITY UNIVERSITYALAGAPPA [Accredited with ’A+’ Grade by NAAC (CGPA:3.64) in the Third Cycle Cycle Third the in (CGPA:3.64) [AccreditedNAAC by withGrade ’A+’’A+’ Gradewith by NAAC[Accredited (CGPA:3.64) in the Third Cycle and Graded as Category–I University by MHRD-UGC] MHRD-UGC] by University and Category–I Graded as as Graded Category–I and University by MHRD-UGC] M.Sc. -

In Vitro Selection for Catalytic Activity with Ribosome Display Patrick Amstutz,† Joelle N
Published on Web 07/17/2002 In Vitro Selection for Catalytic Activity with Ribosome Display Patrick Amstutz,† Joelle N. Pelletier,†,‡ Armin Guggisberg,§ Lutz Jermutus,†,⊥ Sandro Cesaro-Tadic,† Christian Zahnd,† and Andreas Plu¨ckthun*,† Biochemisches Institut, UniVersita¨tZu¨rich, Winterthurerstrasse 190, CH-8057 Zu¨rich, Switzerland, and Organisch-Chemisches Institut der UniVersita¨tZu¨rich, Winterthurerstrasse 190, CH-8057 Zu¨rich, Switzerland Received February 8, 2002 Abstract: We report what is, to our knowledge, the first in vitro selection for catalytic activity based on catalytic turnover by using ribosome display, a method which does not involve living cells at any step. RTEM-â-lactamase was functionally displayed on ribosomes as a complex with its encoding mRNA. We designed and synthesized a mechanism-based inhibitor of â-lactamase, biotinylated ampicillin sulfone, appropriate for selection of catalytic activity of the ribosome-displayed â-lactamase. This derivative of ampicillin inactivated â-lactamase in a specific and irreversible manner. Under appropriate selection conditions, active RTEM-â-lactamase was enriched relative to an inactive point mutant over 100-fold per ribosome display selection cycle. Selection for binding, carried out with â-lactamase inhibitory protein (BLIP), gave results similar to selection with the suicide inhibitor, indicating that ribosome display is similarly efficient in catalytic activity and affinity selections. In the future, the capacity to select directly for enzymatic activity using an entirely in vitro process may allow for a significant increase in the explorable sequence space relative to existing strategies. Naturally occurring enzymes catalyze a wide variety of selection methods sample the entire library in a single experi- chemical reactions and are increasingly used in pharmaceutical, mental step. -

In Vivo Mrna Display Enables Large-Scale Proteomics by Next Generation Sequencing
In vivo mRNA display enables large-scale proteomics by next generation sequencing Panos Oikonomoua,b,c,1, Roberto Salatinob, and Saeed Tavazoiea,b,c,1 aDepartment of Biological Sciences, Columbia University, New York, NY 10027; bDepartment of Systems Biology, Columbia University, New York, NY 10032; and cDepartment of Biochemistry and Molecular Biophysics, Columbia University, New York, NY10032 Edited by Jack W. Szostak, Massachusetts General Hospital, Boston, MA, and approved August 25, 2020 (received for review February 11, 2020) Large-scale proteomic methods are essential for the functional between genotype and phenotype, whereby a protein or peptide characterization of proteins in their native cellular context. How- is linked to its encoding nucleic acid. For example, in phage ever, proteomics has lagged far behind genomic approaches in display, the nucleic acid encoding the capsid displayed peptide is scalability, standardization, and cost. Here, we introduce in vivo contained within the phage (33). The resulting collection of mRNA display, a technology that converts a variety of proteomics displayed peptides can be used for the in vitro characterization of applications into a DNA sequencing problem. In vivo-expressed protein interactions, protein engineering, and selection of human proteins are coupled with their encoding messenger RNAs antibody fragment libraries (34–36). Alternative in vitro methods (mRNAs) via a high-affinity stem-loop RNA binding domain inter- linking nucleotide information to phenotype include mRNA action, enabling high-throughput identification of proteins with display (37, 38), ribosome display (39), and yeast display (40). In high sensitivity and specificity by next generation DNA sequenc- the past decade, many of these technologies have been coupled ing. -

Improving Protein Therapeutics Through Quantitative Molecular Engineering Approaches and a Cell-Based Oral Delivery Platform
University of Pennsylvania ScholarlyCommons Publicly Accessible Penn Dissertations 2013 Improving Protein Therapeutics Through Quantitative Molecular Engineering Approaches and A Cell-Based Oral Delivery Platform Ting Wun Ng University of Pennsylvania, [email protected] Follow this and additional works at: https://repository.upenn.edu/edissertations Part of the Biomedical Commons Recommended Citation Ng, Ting Wun, "Improving Protein Therapeutics Through Quantitative Molecular Engineering Approaches and A Cell-Based Oral Delivery Platform" (2013). Publicly Accessible Penn Dissertations. 784. https://repository.upenn.edu/edissertations/784 This paper is posted at ScholarlyCommons. https://repository.upenn.edu/edissertations/784 For more information, please contact [email protected]. Improving Protein Therapeutics Through Quantitative Molecular Engineering Approaches and A Cell-Based Oral Delivery Platform Abstract Proteins, with their ability to perform a variety of highly specific biological functions, have emerged as an important class of therapeutics. However, to fully harness their therapeutic potential, proteins often need to be optimized by molecular engineering; therapeutic efficacy can be improved by modulating protein properties such as binding affinity/specificity, half-life, bioavailability, and immunogenicity. In this work, we first present an introductory example in which a mechanistic mathematical model was used to improve target selection for directed evolution of an aglycosylated Fc domain of an antibody to enhance -

EURL ECVAM Recommendation on Non-Animal-Derived Antibodies
EURL ECVAM Recommendation on Non-Animal-Derived Antibodies EUR 30185 EN Joint Research Centre This publication is a Science for Policy report by the Joint Research Centre (JRC), the European Commission’s science and knowledge service. It aims to provide evidence-based scientific support to the European policymaking process. The scientific output expressed does not imply a policy position of the European Commission. Neither the European Commission nor any person acting on behalf of the Commission is responsible for the use that might be made of this publication. For information on the methodology and quality underlying the data used in this publication for which the source is neither Eurostat nor other Commission services, users should contact the referenced source. EURL ECVAM Recommendations The aim of a EURL ECVAM Recommendation is to provide the views of the EU Reference Laboratory for alternatives to animal testing (EURL ECVAM) on the scientific validity of alternative test methods, to advise on possible applications and implications, and to suggest follow-up activities to promote alternative methods and address knowledge gaps. During the development of its Recommendation, EURL ECVAM typically mandates the EURL ECVAM Scientific Advisory Committee (ESAC) to carry out an independent scientific peer review which is communicated as an ESAC Opinion and Working Group report. In addition, EURL ECVAM consults with other Commission services, EURL ECVAM’s advisory body for Preliminary Assessment of Regulatory Relevance (PARERE), the EURL ECVAM Stakeholder Forum (ESTAF) and with partner organisations of the International Collaboration on Alternative Test Methods (ICATM). Contact information European Commission, Joint Research Centre (JRC), Chemical Safety and Alternative Methods Unit (F3) Address: via E. -

Chapter 1 Ribosome Display
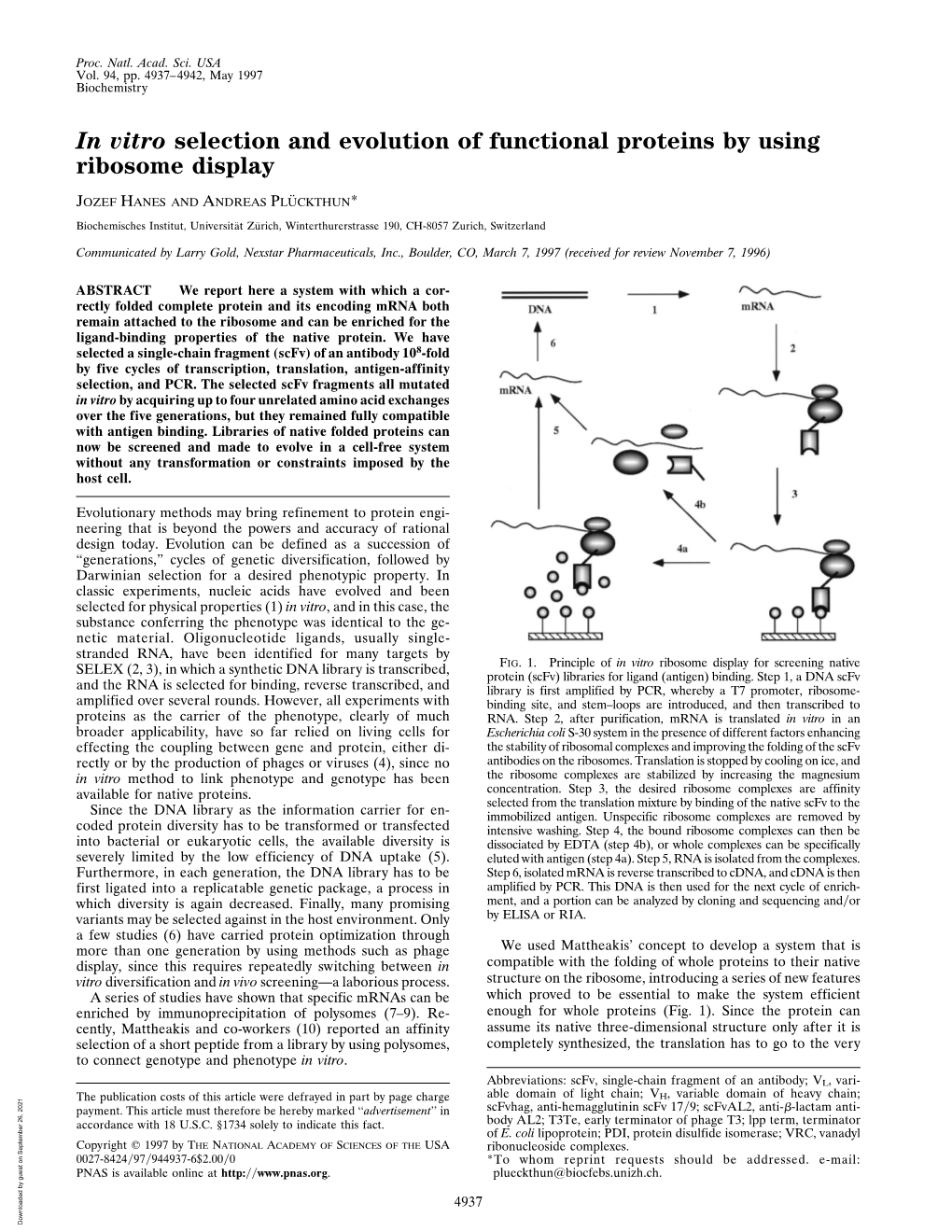
Chapter 1 Ribosome Display: A Perspective Andreas Plückthun Abstract Ribosome display is an in vitro evolution technology for proteins. It is based on in vitro translation, but prevents the newly synthesized protein and the mRNA encoding it from leaving the ribosome . It thereby couples phenotype and genotype. Since no cells need to be transformed, very large libraries can be used directly in selections, and the in vitro amplifi cation provides a very convenient integration of random mutagenesis that can be incorporated into the procedure. This review highlights concepts, mechanisms, and different variations of ribosome display and compares it to related methods. Applications of ribosome display are summarized, e.g., the directed evolution of proteins for higher binding affi nity, for higher stability or other improved biophysical parameters and enzymatic properties. Ribosome display has developed into a robust technology used in academia and industry alike, and it has made the cell-free Darwinian evolution of proteins over multiple generations a reality. Key words: Directed evolution , Cell-free translation, Ribosome display , Protein engineering, Antibody engineering , DARPins , Designed ankyrin repeat proteins , Affi nity maturation 1. Introduction: Ribosome Display in Context All technologies of molecular evolution must couple phenotype and genotype. There are two fundamental possibilities for achieving this. The fi rst one is compartmentalization. Nature’s compartments are cells: they secure that the superior phenotype expressed by one cell’s mutant genotype can be replicated, without the gene products from the wild type interfering. All selections based on microbial phenotypes use this principle. The second possibility is a direct physical coupling of genetic material to the protein product. -

Evolution of Translation Initiation Sequences Using in Vitro Yeast Ribosome Display
ARTICLE Evolution of Translation Initiation Sequences Using In Vitro Yeast Ribosome Display Rui Gan,1 Michael C. Jewett1,2,3,4 1 Department of Chemical and Biological Engineering, Northwestern University, 2145 Sheridan Road, Evanston, Illinois 60208 2 Chemistry of Life Processes Institute, Northwestern University, Evanston, Illinois 3 Member, Robert H. Lurie Comprehensive Cancer Center, Northwestern University, Evanston, Illinois 4 Simpson Querrey Institute, Northwestern University, Evanston, Illinois; fax: 847-491-3728; e-mail: [email protected] Introduction ABSTRACT: We report a novel in vitro yeast ribosome display method based on cell-free protein synthesis (CFPS) using linear The ability to regulate gene expression is critical to bioengineering DNA templates. We demonstrate that our platform can enrich a applications. Over the last few decades, many different regulatory target gene from a model library by 100-fold per round of selection. strategies have been used to control the expression of toxic proteins We demonstrate the utility of our approach by evolving cap- in hosts, to maximize yield of recombinant proteins, and to tune the independent translation initiation (CITI) sequences, which result in expression of key enzymes in metabolic pathways in order to a 13-fold increase in CFPS yields after four rounds of selection, and fi balance the flux of metabolites (Farmer and Liao, 2000; Jones et al., a threefold further increase by placing the bene cial short fi sequences in tandem. We also show that 12 of the selected CITI 2000; Kim and Keasling, 2001). Recently, rapid progress in the eld sequences permit precise control of gene expression in vitro over a of synthetic biology has enabled de novo metabolic pathway design, range of up to 80-fold by enhancing translation (and not as cryptic genetic circuit construction, and artificial genome synthesis promoters). -

Chapter 2 Mrna Display
8 Chapter 2 mRNA Display: Ligand Discovery, Interaction Analysis and Beyond This work has been adapted from the following publication: Takahashi, T.T., Austin, R.J. and Roberts, R.W. mRNA display: ligand discovery, interaction analysis and beyond. (2003) Trends Biochem Sci 28, 159-165. 9 Abstract In vitro peptide and protein selection using mRNA display* enables the discovery and directed evolution of new molecules from combinatorial libraries. These selected molecules can serve as tools to control and understand biological processes, enhance our understanding of molecular interactions, and potentially treat disease in therapeutic applications. In mRNA display, mRNA molecules are covalently attached to the peptide or protein they encode. These mRNA-protein fusions enable in vitro selection of peptide and protein libraries of more than 1013 different sequences. mRNA display has been used to discover novel peptide and protein ligands for RNA, small molecules, and proteins, as well as to define cellular interaction partners of proteins and drugs. In addition, several unique applications are possible with mRNA display, including self-assembling protein chips and library construction with unnatural amino acids, and chemically modified peptides. *mRNA display has been referred to as mRNA-protein fusions (1), in vitro virus and in vitro virus virion (2), and PROfusionTM technology (3). 10 Introduction Functional approaches, such as in vitro selection, currently provide the best means available for isolating peptides and proteins with desired chemical or biochemical properties. Over the last decade, display technologies have been essential tools in the discovery of peptide and protein ligands and in delineating in vivo interaction partners. The phage (4) and ribosome display systems (5) have been principally used for discovery, while the yeast two-hybrid method (6) has been used for in vivo interaction analysis. -

Cell Surface Display
40 Cell Surface Display Sharadwata Pan and Michael K. Danquah Monash University Australia 1. Introduction The manipulation of the cell surfaces of prokaryotes (mainly bacteria) and eukaryotes (such as Yeast) has manifested to be an area of stupendous ongoing research, with intelligent widespread applications spanning different arenas of biological sciences (Charbit et al., 1988; Cruz et al., 2000; Francisco et al., 1993; Götz, 1990; Jostock & Dübel, 2005; Keskinkan et al., 2004; Kotrba et al., 1999; Lee & Schnaitman, 1980; Liljeqvist et al., 1997; Martineau et al., 1991; Mizuno et al., 1983; Sousa et al., 1996; Taschner et al., 2002; Wernérus & Ståhl, 2004; Willett et al., 1995; Xu & Lee, 1999). Till date, majority of the surface display systems developed for Gram-negative bacteria involve introducing external peptides into surface-approachable loops of naturally displayed proteins. This sometimes put extreme size restrictions on the displayed components (Wernérus & Ståhl, 2004). However, this problem is more or less resolved since larger proteins could be inserted through some recently developed bacterial display systems for Gram-negative bacteria (Charbit et al., 1988; Cruz et al., 2000; Lee & Schnaitman, 1980; Mizuno et al., 1983; Xu & Lee, 1999). Thanks to some tireless research, it is now evident that the structural properties of the cell wall in Gram-positive bacteria, i.e. the thick peptidoglycan layer, make them suitable candidates for strict laboratory procedures and demanding field applications (Jostock & Dübel, 2005). On the other hand, lower transformation efficiency has been a significant disadvantage of using Gram-positive bacteria (Wernérus & Ståhl, 2004), considering if someone is working with surface-displayed conjunctional libraries for affinity-based selections. -

In Vitro Selection of Peptides and Proteins Advantages of Mrna
pubs.acs.org/synthbio Review In Vitro Selection of Peptides and ProteinsAdvantages of mRNA Display Matilda S. Newton, Yari Cabezas-Perusse, Cher Ling Tong, and Burckhard Seelig* Cite This: https://dx.doi.org/10.1021/acssynbio.9b00419 Read Online ACCESS Metrics & More Article Recommendations ABSTRACT: mRNA display is a robust in vitro selection technique that allows the selection of peptides and proteins with desired functions from libraries of trillions of variants. mRNA display relies upon a covalent linkage between a protein and its encoding mRNA molecule; the power of the technique stems from the stability of this link, and the large degree of control over experimental conditions afforded to the researcher. This article describes the major advantages that make mRNA display the method of choice among comparable in vivo and in vitro methods, including cell-surface display, phage display, and ribosomal display. We also describe innovative techniques that harness mRNA display for directed evolution, protein engineering, and drug discovery. KEYWORDS: protein engineering, in vitro selection, mRNA display, phage display, unnatural amino acids, ribosome display mRNA display is an in vitro selection and directed evolution technique that enables the screening of trillions of protein variants for desired functions in a single experiment. In directed evolution, researchers working to alter binding or catalytic properties of a target protein or peptide have a range of in vitro and in vivo techniques at their disposal to isolate their desired variant from large mixtures of variants. The goal of this review is to highlight the unique advantages of mRNA display that make this method superior to most other directed evolution techniques. -

Evolutives Protein-Design Eines „Anticalins“ Mit Bindungsspezifität Für Digoxigenin
Lehrstuhl für Biologische Chemie Forschungsdepartment für Biowissenschaftliche Grundlagen Evolutives Protein-Design eines „Anticalins“ mit Bindungsspezifität für Digoxigenin Dipl.-Ing. Steffen Schlehuber Vollständiger Abdruck der von der Fakultät Wissenschaftszentrum Weihenstephan für Ernährung, Landnutzung und Umwelt der Technischen Universität München zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften genehmigten Dissertation. Vorsitzender: Univ.-Prof. Dr. J. Friedrich Prüfer der Dissertation: 1. Univ.-Prof. Dr. A. Skerra 2. Univ.-Prof. Dr. H. Klostermeyer, emeritiert Die Dissertation wurde am 12.10.2001 bei der Technischen Universität München eingereicht und durch die Fakultät Wissenschaftszentrum Weihenstephan für Ernährung, Landnutzung und Umwelt am 06.12.2001 angenommen. Die vorliegende Arbeit wurde in der Abteilung Proteinchemie am Institut für Biochemie, Fachbereich Chemie, der Technischen Universität Darmstadt begonnen und am Lehrstuhl für Biologische Chemie im Forschungsdepartment für Biowissenschaftliche Grundlagen am Wissenschaftszentrum Weihenstephan für Ernährung, Landnutzung und Umwelt der Techni- schen Universität München weitergeführt und abgeschlossen. Herrn Prof. Dr. A. Skerra danke ich für die interessante und vielseitige Themenstellung, für zahlreiche inspirierende Diskussionen sowie das stete Interesse am Fortgang und Erfolg meiner Arbeit. Meinen Kolleginnen und Kollegen der Abteilung Proteinchemie und des Lehrstuhls für Biolo- gische Chemie danke ich für ihre Hilfsbereitschaft und das ausgezeichnete