Epigenetics, Environment and Health Research Consortium Network
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
SM08 Programme
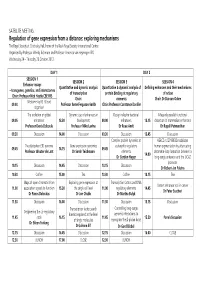
SATELLITE MEETING Regulation of gene expression from a distance: exploring mechanisms The Royal Society at Chicheley Hall, home of the Kavli Royal Society International Centre Organised by Professor Wendy Bickmore and Professor Veronica van Heyningen FRS Wednesday 24 – Thursday 25 October 2012 DAY 1 DAY 2 SESSION 1 SESSION 2 SESSION 3 SESSION 4 Enhancer assays Quantitative and dynamic analysis Quantitative & dynamic analysis of Defining enhancers and their mechanisms – transgenes, genetics, and interactomes of transcription protein binding at regulatory of action Chair: Professor Nick Hastie CBE FRS Chair: elements Chair: Dr Duncan Odom Welcome by RS & lead 09.00 Professor Anne Ferguson-Smith Chair: Professor Constance Bonifer organiser The evolution of global Dynamic use of enhancers in Design rules for bacterial Massively parallel functional 09.05 enhancers 13.30 development 09.00 enhancers 13.15 dissection of mammalian enhancers Professor Denis Duboule Professor Mike Levine Dr Roee Amit Dr Rupali Patwardhan 09.30 Discussion 14.00 Discussion 09.30 Discussion 13.45 Discussion Complex protein dynamics at HERC2 rs12913832 modulates The pluripotent 3D genome Gene expression genomics eukaryotic regulatory human pigmentation by attenuating 09.45 14.15 09.45 Professor Wouter de Laat Dr Sarah Teichmann elements chromatin-loop formation between a 14.00 Dr Gordon Hager long-range enhancer and the OCA2 promoter 10.15 Discussion 14.45 Discussion 10.15 Discussion Dr Robert-Jan Palstra 10.30 Coffee 15.00 Tea 10.30 Coffee 14.15 Tea Maps of open chromatin -

Epigenetics of the Synapse in Neurodegeneration
Current Neurology and Neuroscience Reports (2019) 19:72 https://doi.org/10.1007/s11910-019-0995-y GENETICS (V. BONIFATI, SECTION EDITOR) Epigenetics of the Synapse in Neurodegeneration Mary Xylaki1 & Benedict Atzler1 & Tiago Fleming Outeiro1,2,3 # The Author(s) 2019 Abstract Purpose of Review In the quest for understanding the pathophysiological processes underlying degeneration of nervous systems, synapses are emerging as sites of great interest as synaptic dysfunction is thought to play a role in the initiation and progression of neuronal loss. In particular, the synapse is an interesting target for the effects of epigenetic mechanisms in neurodegeneration. Here, we review the recent advances on epigenetic mechanisms driving synaptic compromise in major neurodegenerative disorders. Recent Findings Major developments in sequencing technologies enabled the mapping of transcriptomic patterns in human postmortem brain tissues in various neurodegenerative diseases, and also in cell and animal models. These studies helped identify changes in classical neurodegeneration pathways and discover novel targets related to synaptic degeneration. Summary Identifying epigenetic patterns indicative of synaptic defects prior to neuronal degeneration may provide the basis for future breakthroughs in the field of neurodegeneration. Keywords Synapse . Neurodegenerative diseases . DNA methylation . Histone modifications . Noncoding RNAs . Neuroepigenetics Abbreviations CpG Cytosine-phosphate-guanine 3xTg-AD Triple-transgenic AD CpH Non-CpG 5xFAD Five familial -

Neuroepigenetics: Introduction to the Special Issue on Epigenetics in Neurodevelopment and Neurological Diseases
Experimental Neurology 268 (2015) 1–2 Contents lists available at ScienceDirect Experimental Neurology journal homepage: www.elsevier.com/locate/yexnr Editorial Neuroepigenetics: Introduction to the special issue on epigenetics in neurodevelopment and neurological diseases Gene expression profile represents the molecular state of a cell at a The structural unit of the chromatin is nucleosome, which is com- given time and is dynamically regulated and precisely controlled in re- prised of DNA chains wrapping around histone proteins. Nucleosomes sponse to external stimuli. While the DNA sequences are the ultimate can be either loosely or densely packed, which is associated with active templates for gene transcription, epigenetic features of the genome, in- or suppressed gene transcription, respectively. The chemical modifica- cluding modifications of histones, methylation of cytosine nucleotides tions of the histone tail, most commonly acetylation, methylation and of the genomic DNA, 3-dimensional interaction of genomic regions, phosphorylation, are one of the determinants of the structural status and the expression of various forms of non-coding RNAs, regulate the of the chromatin. Histone acetylation is in general correlates with accessibility and processability of the genomic loci and add another loose chromatin and active gene expression. On the other hand, layer of regulation of gene expression beyond the heritable DNA se- Histone-3 lysine-4 trimethylation (H3K4Me3) plays both positive and quences. For decades, some epigenetic properties of the genome, such negative roles in regulating gene expression, depending on the combi- as DNA methylation, have been considered as stable and inheritable nation of other histone modifications. The article by Shen and col- marks. -

CAMB713: Neuroepigenetics
CAMB713: Neuroepigenetics TIME: Thursdays 1-3pm 8/29 – 12/12 (organizational meeting 8/29, no class on 9/5 and 11/28) LOCATION: BRB 1413 COURSE DIRECTORS: Zhaolan (Joe) Zhou 215.746.5025 [email protected] Elizabeth Heller 215.573.7038 [email protected] Hao Wu 215.573.9360 [email protected] GOALS: This is a course intended to bring students up to date concerning our understanding of Neural Epigenetics. It is based on assigned topics and readings covering a variety of experimental systems and concepts in the field of Neuroepigenetics, formal presentations by individual students, critical evaluation of primary data, and in-depth discussion of potential issues and future directions, with goals to: 1) Review basic concepts of epigenetics in the context of neuroscience 2) Learn to critically evaluate a topic (not a single paper) and rigor of prior research 3) Improve experimental design and enhance rigor and reproducibility 4) Catch up with the most recent development in neuroepigenetics 5) Develop professional presentation skills - be a story teller FORMAT: Each week will focus on a specific topic of Neuroepigenetics via a “seminar” style presentation by a class member with the following expectations: Consultation with preceptor prior to presentation Introduction (~20 min): Context of topic in the field Historic perspectives of the topic Current understandings Primary data (~40 min): Questions of interest Design of experiments Interpretation of data Discussion (~20 min): Issues/challenges Proposed future -

High-Resolution Mapping of Transcriptional Dynamics Across Tissue Development Reveals a Stable Mrna–Trna Interface
Downloaded from genome.cshlp.org on September 30, 2021 - Published by Cold Spring Harbor Laboratory Press Research High-resolution mapping of transcriptional dynamics across tissue development reveals a stable mRNA–tRNA interface Bianca M. Schmitt,1,5 Konrad L.M. Rudolph,2,5 Panagiota Karagianni,3 Nuno A. Fonseca,2 Robert J. White,4 Iannis Talianidis,3 Duncan T. Odom,1 John C. Marioni,2 and Claudia Kutter1 1University of Cambridge, Cancer Research UK Cambridge Institute, Cambridge, CB2 0RE, United Kingdom; 2European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Trust Genome Campus, Hinxton, Cambridge, CB10 1SD, United Kingdom; 3B.S.R.C. Alexander Flemming, 16672, Vari, Athens, Greece; 4University of York, Department of Biology, Heslington, York, YO10 5DD, United Kingdom The genetic code is an abstraction of how mRNA codons and tRNA anticodons molecularly interact during protein synthesis; the stability and regulation of this interaction remains largely unexplored. Here, we characterized the expression of mRNA and tRNA genes quantitatively at multiple time points in two developing mouse tissues. We dis- covered that mRNA codon pools are highly stable over development and simply reflect the genomic background; in contrast, precise regulation of tRNA gene families is required to create the corresponding tRNA transcriptomes. The dynamic regulation of tRNA genes during development is controlled in order to generate an anticodon pool that closely corresponds to messenger RNAs. Thus, across development, the pools of mRNA codons and tRNA anticodons are invariant and highly correlated, revealing a stable molecular interaction interlocking transcription and translation. [Supplemental material is available for this article.] Transcription of the mammalian genome is divided among mul- differencesinexpressionofspecifictRNAgenesmaycontribute tiple RNA polymerases (Pol), each transcribing a nonoverlapping to tumorigenesis by favoring translation of cancer-promoting set of genes. -

DKFZ Postdoctoral Fellowships 2021 Hosting Group Information For
DKFZ Postdoctoral Fellowships 2021 Hosting group information for applicants Name of DKFZ research division/group: Division of Regulatory Genomics and Cancer Evolution (B270) Contact person: Duncan Odom Group homepage: https://www.dkfz.de/en/regulatorische-genomik/index.php Please visit our website for further information on our research and recent publications. RESEARCH PROFILE AND PROJECT TOPICS: Dr Odom’s laboratory studies how genetic sequence information shapes the cell's DNA regulatory landscape and thus the trajectory of cancer genome evolution. Our long-standing interest in interspecies analysis of matched functional genomic data has revealed the extensive and rapid turn-over of tissue-specific transcription factor binding, insulator elements, polymerase occupancies, and enhancer activities during organismal evolution. My laboratory has begun exploiting single-cell RNA-sequencing and large-scale whole genome sequencing in understanding molecular and cancer genome evolution. Recent high-profile studies from the Odom lab used single-cell transcriptional analysis to conclusively demonstrate that ageing results in substantial increases in cell-to-cell transcriptional variability and exploited chemical carcinogenesis in mouse liver to reveal how the persistence of DNA lesions profoundly shapes tumour genomes. First, we are currently interviewing for experimentally focused postdoctoral fellows to spearhead multiple diverse and interdisciplinary collaborations in single-cell analysis, functional genomics, and spatial transcriptomics with Gerstung, Stegle, Hoefer, and Goncalves laboratories at DKFZ. Such an appointment would offer unusual opportunities for an experimentally-focused postdoc to learn and deploy bioinformatics tools in collaboration with one or more world-leading computational biology laboratories. Second, we are seeking a postdoctoral researcher to spearhead a collaboration with Edith Heard's group at EMBL, focused on how X-chromosome dynamics can shape disease susceptibility in older individuals. -

Contribution of Neuroepigenetics to Huntington's Disease
Francelle L, Lotz C, Outeiro T, Brouillet E, Merienne K. Contribution of Neuroepigenetics to Huntington’s Disease. Frontiers in Human Neuroscience 2017, 11, 17. Copyright: © 2017 Francelle, Lotz, Outeiro, Brouillet and Merienne. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms. DOI link to article: https://doi.org/10.3389/fnhum.2017.00017 Date deposited: 19/12/2017 This work is licensed under a Creative Commons Attribution 4.0 International License Newcastle University ePrints - eprint.ncl.ac.uk fnhum-11-00017 January 25, 2017 Time: 15:35 # 1 REVIEW published: 30 January 2017 doi: 10.3389/fnhum.2017.00017 Contribution of Neuroepigenetics to Huntington’s Disease Laetitia Francelle1*, Caroline Lotz2, Tiago Outeiro1, Emmanuel Brouillet3 and Karine Merienne2 1 Department of NeuroDegeneration and Restorative Research, University Medical Center Goettingen, Goettingen, Germany, 2 CNRS UMR 7364, Laboratory of Cognitive and Adaptive Neurosciences, University of Strasbourg, Strasbourg, France, 3 Commissariat à l’Energie Atomique et aux Energies Alternatives, Département de Recherche Fondamentale, Institut d’Imagerie Biomédicale, Molecular Imaging Center, Neurodegenerative diseases Laboratory, UMR 9199, CNRS Université Paris-Sud, Université Paris-Saclay, Fontenay-aux-Roses, France Unbalanced epigenetic regulation is thought to contribute to the progression of several neurodegenerative diseases, including Huntington’s disease (HD), a genetic disorder considered as a paradigm of epigenetic dysregulation. -

Paterson Institute for Cancer Research Scientific Report 2008 Contents
paterson institute for cancer research scientific report 2008 cover images: Main image supplied by Karim Labib and Alberto sanchez-Diaz (cell cycle Group). Budding yeast cells lacking the inn1 protein are unable to complete cytokinesis. these cells express a fusion of a green fluorescent protein to a marker of the plasma membrane, and have red fluorescent proteins attached to components of the spindle poles and actomyosin ring (sanchez-Diaz et al., nature cell Biology 2008; 10: 395). Additional images: front cover image supplied by Helen rushton, simon Woodcock and Angeliki Malliri (cell signalling Group). the image is of a mitotic spindle in fixed MDcK (Madin-Darby canine kidney) epithelial cells, which have been stained with an anti-beta tubulin antibody (green), DApi (blue) and an anti-centromere antibody (crest, red) which recognises the kinetochores of the chromosomes. the image was taken on the spinning disk confocal microscope using a 150 x lens. rear cover image supplied by Andrei ivanov and tim illidge (targeted therapy Group). Visualisation of tubulin (green) and quadripolar mitosis (DnA stained with DApi), Burkitt’s lymphoma namalwa cell after 10 Gy irradiation. issn 1740-4525 copyright 2008 © cancer research UK Paterson Institute for Cancer Research Scientific Report 2008 Contents 4 Director’s Introduction Researchers’ pages – Paterson Institute for Cancer Research 8 Crispin Miller Applied Computational Biology and Bioinformatics 10 Geoff Margison Carcinogenesis 12 Karim Labib Cell Cycle 14 Iain Hagan Cell Division 16 Nic Jones -

About Microneurotrophins
About MicroNeurotrophins UNLOCKING THE PROMISE OF NEUROTROPHINS Most researchers now agree that ALS and other neurodegenerative diseases including Multiple Sclerosis, Parkinson’s and Alzheimer’s are remarkably complex, almost certainly involving a combination of different biological, genetic and external triggers. Yet, time and again, drug development for these multi-faceted diseases has focused almost exclusively on medications that only target one factor likely causing the disease. It’s not surprising that these single-target drugs have uniformly failed in previous clinical trials. With this in mind, esteemed pharmacologist Dr. Achilleas Gravanis and his team of scientists from Bionature, a spin-off of the University of Crete in Greece, began searching for a way to effectively address the multiple triggers contributing to the onset and progression of neurodegenerative diseases. Their investigation led them to study neurotrophins, proteins naturally secreted in the brain that transmit signals between motor neurons by binding to and activating two different types of receptors, TrkA and p75, found on the surface of nerve cells. Twenty years of research have shown that neurotrophins help motor neurons develop, grow, function, signal and even repair themselves. Neurotrophins also appear to protect motor neurons from stress and death. By contrast, ALS causes motor neurons to degenerate and die and ALS patients experience a profound loss of neurotrophins as the disease progresses. However, numerous clinical trials have been unable to prove the efficacy of neurotrophins to treat ALS and other neurodegenerative diseases. TWO STARTLING DISCOVERIES To understand why, Dr. Gravanis and his colleagues carefully examined past studies on neurotrophins, and two astonishing observations became apparent to them. -

Register of Declared Private, Professional Commercial and Other Interests
REGISTER OF DECLARED PRIVATE, PROFESSIONAL COMMERCIAL AND OTHER INTERESTS Please refer to the attached guidance notes before completing this register entry. In addition to guidance on each section, examples of information required are also provided. Where you have no relevant interests in the relevant category, please enter ‘none’ in the register entry. Name: Michaela Frye Please list all MRC bodies you are a member of: E.g. Council, Strategy Board, Research Board, Expert Panel etc and your position (e.g. chair, member). Regenerative Medicine Research Committee member Main form of employment: Name of University and Department or other employing body (include location), and your position. University of Cambridge, Department of Genetics, Cancer Research UK Senior Fellow Research group/department web page: Provide a link to any relevant web pages for your research group or individual page on your organisation’s web site. http://www.stemcells.cam.ac.uk/research/affiliates/dr-frye http://www.gen.cam.ac.uk/research-groups/frye Please give details of any potential conflicts of interests arising out of the following: 1. Personal Remuneration: Including employment, pensions, consultancies, directorships, honoraria. See section 1 for further guidance. None 2. Shareholdings and Financial Interests in companies: Include the names of companies involved in medical/biomedical research, pharmaceuticals, biotechnology, healthcare provision and related fields where shareholdings or other financial interests. See section 2 for thresholds and further guidance. None 3. Research Income during current session (financial year) : Declare all research income from bodies supported by the MRC and research income from other sources above the limit of £50k per grant for the year. -

Transcription Factor Binding Cells and Their Progeny
RESEARCH HIGHLIGHTS knock-in alleles at the Lgr6 locus, allowing them to visualize Lgr6+ Transcription factor binding cells and their progeny. Shortly after birth, Lgr6 expression in the skin becomes restricted, eventually marking a unique population located at Most transcription factor binding events differ between closely the central isthmus just above the bulge region in resting hair follicles. related species despite conservation of functional targets. Now, Paul Flicek and Duncan Odom and colleagues report an Through a series of lineage-tracing studies in adult mice, they showed + evolutionary comparison of transcription factor CEBPA binding that the progeny of Lgr6 cells contribute not only to hair follicles but among five vertebrate species (Science published online, also to sebaceous glands and interfollicular epidermis. They also showed + doi:10.1126/science.1186176, 8 April 2010). The authors that transplanted Lgr6 stem cells could reconstitute fully formed hair compared genome-wide binding of CEBPA as determined by follicles and contribute to all three lineages in host skin. Finally, they + ChIP-sequencing in livers of human, mouse, dog, short-tailed showed that Lgr6 cells contribute to wound healing. These findings opossum and chicken. The CEBPA binding motif was virtually identify Lgr6 as a marker of a distinct epidermal stem cell population identical between the five species, but most binding events contributing to all three skin lineages. KV were species-specific. Only 10% to 22% of binding events were shared between any two placental mammals, and there was even greater divergence of binding events in opossum and chicken. Identifying nonobvious phenologs Only 35 binding events were shared by all five species. -

Psychotropic Drug-Induced Genetic- Epigenetic Modulation of CRTC1
Delacrétaz et al. Clinical Epigenetics (2019) 11:198 https://doi.org/10.1186/s13148-019-0792-0 RESEARCH Open Access Psychotropic drug-induced genetic- epigenetic modulation of CRTC1 gene is associated with early weight gain in a prospective study of psychiatric patients Aurélie Delacrétaz1, Anaïs Glatard1, Céline Dubath1, Mehdi Gholam-Rezaee2, Jose Vicente Sanchez-Mut3, Johannes Gräff3, Armin von Gunten4, Philippe Conus5 and Chin B. Eap1,6* Abstract Background: Metabolic side effects induced by psychotropic drugs represent a major health issue in psychiatry. CREB-regulated transcription coactivator 1 (CRTC1) gene plays a major role in the regulation of energy homeostasis and epigenetic mechanisms may explain its association with obesity features previously described in psychiatric patients. This prospective study included 78 patients receiving psychotropic drugs that induce metabolic disturbances, with weight and other metabolic parameters monitored regularly. Methylation levels in 76 CRTC1 probes were assessed before and after 1 month of psychotropic treatment in blood samples. Results: Significant methylation changes were observed in three CRTC1 CpG sites (i.e., cg07015183, cg12034943, and cg 17006757) in patients with early and important weight gain (i.e., equal or higher than 5% after 1 month; FDR p value = 0.02). Multivariable models showed that methylation decrease in cg12034943 was more important in patients with early weight gain (≥ 5%) than in those who did not gain weight (p = 0.01). Further analyses combining genetic and methylation data showed that cg12034943 was significantly associated with early weight gain in patients carrying the G allele of rs4808844A>G (p = 0.03), a SNP associated with this methylation site (p =0.03).