Quantity Limits—Premium
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Crofelemer Oral Delayed Release Tablet
Contains Nonbinding Recommendations Draft – Not for Implementation Draft Guidance on Crofelemer This draft guidance, when finalized, will represent the current thinking of the Food and Drug Administration (FDA, or the Agency) on this topic. It does not establish any rights for any person and is not binding on FDA or the public. You can use an alternative approach if it satisfies the requirements of the applicable statutes and regulations. To discuss an alternative approach, contact the Office of Generic Drugs. Active Ingredient: Crofelemer Dosage Form; Route: Tablet, delayed release; oral Strength: 125 mg Recommendations for the Assessment of Identity and Quality of Botanical Raw Material (BRM): Crofelemer is a botanical drug derived from BRM, the crude red latex of Croton lechleri Müll. Arg. [Fam. Euphorbiacae], which is also called dragon’s blood (sangre de drago). Generic drug applicants should use the same plant species and perform BRM assessment: 1. Crofelemer BRM should be collected from the crude red latex of Croton lechleri. The plant species should be correctly identified and authenticated based on techniques such as macroscopic/microscopic and/or analysis of genetic material. 2. Crude red latex as BRM should be collected from the mature tree with defined eco- geographic regions (EGRs). Implementing and enforcing established good agricultural and collection practice (GACP) procedures will minimize variations in BRM and ensure batch- to-batch consistency of crofelemer. 3. BRMs should be analyzed for their crofelemer content, total phenolics and taspine content, as well as heavy metals and pesticides. Recommendations for Demonstrating API Sameness: API sameness can be established by showing equivalence between Test API and API from the reference listed drug (RLD) product with the three criteria described in detail below. -

Summary of Drug Limitations Mary C
RON DESANTIS GOVERNOR SUMMARY OF DRUG LIMITATIONS MARY C. MAYHEW SECRETARY **Medications listed in this document may or may not require a prior authorization. Please view the Preferred Drug List at: http://ahca.myflorida.com/Medicaid/Prescribed_Drug/pharm_thera/fmpdl.shtml** Summary of Drug Limitations Abilify (aripiprazole) 2mg, 5mg, 20mg, 30mg tablets Minimum age = 6; Maximum of 1 tablet per day Abilify (aripiprazole) 10mg, 15mg tablets Minimum age = 6; Maximum of 15mg per day for ages = 6 - 11; Maximum of 30mg per day for ages = 12-17 Maximum of 1 tablet per day Abilify (aripiprazole) Discmelt 10mg, 15mg tabs Minimum age = 6; Maximum of 15mg per day for ages = 6 - 11; Maximum of 30mg per day for ages = 12-17; Maximum of 2 tablets per day Abilify (aripiprazole) 1mg/ml solution Minimum age = 6; Maximum of 15ml per day for ages = 6 - 11; Maximum of 30ml per day for ages = 12-17; Maximum of 30ml per day for ages =/> 18 Abilify Maintena (aripiprazole) syringe/vial Minimum age = 18; Maximum of 1 syringe or vial every 28 days Absorica (isotretinoin) 10mg, 20mg, 25mg,30mg, 35mg, & Minimum age = 12 40mg capsules Abstral (fentanyl citrate) sublingual tablets Minimum age = 18; Maximum of 4 sublingual tablets per day Acanya (benzoyl peroxide/clindamycin)Gel, gel pump Minimum Age= 12 Accolate (zafirlukast) tablets Maximum of 3 tablets per day Aciphex (rabeprazole) 5mg, 10mg sprinkle capsules Minimum age = 1; Maximum age = 11; Maximum of 1 capsule per day Aciphex (rabeprazole) 20mg tablets Minimum age = 1; Maximum of 2 tablets per day Actemra (tocilizumab) 80mg/4ml, 200mg/10ml, Minimum age= 2 400mg/20ml Vials, & 162mg/0.9ml Syringe Actimmune (Interferon Gamma-1b) Maximum of 6ml every 28days Actiq (fentanyl citrate) Lozenges Minimum age = 18; Maximum of 4 lozenges per day Activella (estradiol/norethindrone) tablets Minimum age = 18 Updated 02/28/2019 1 RON DESANTIS GOVERNOR SUMMARY OF DRUG LIMITATIONS MARY C. -

XERMELO™ (Telotristat Ethyl)
PHARMACY COVERAGE GUIDELINES ORIGINAL EFFECTIVE DATE: 5/18/2017 SECTION: DRUGS LAST REVIEW DATE: 5/20/2021 LAST CRITERIA REVISION DATE: 5/20/2021 ARCHIVE DATE: XERMELO™ (telotristat ethyl) Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy Coverage Guideline must be read in its entirety to determine coverage eligibility, if any. This Pharmacy Coverage Guideline provides information related to coverage determinations only and does not imply that a service or treatment is clinically appropriate or inappropriate. The provider and the member are responsible for all decisions regarding the appropriateness of care. Providers should provide BCBSAZ complete medical rationale when requesting any exceptions to these guidelines. The section identified as “Description” defines or describes a service, procedure, medical device or drug and is in no way intended as a statement of medical necessity and/or coverage. The section identified as “Criteria” defines criteria to determine whether a service, procedure, medical device or drug is considered medically necessary or experimental or investigational. State or federal mandates, e.g., FEP program, may dictate that any drug, device or biological product approved by the U.S. Food and Drug Administration (FDA) may not be considered experimental or investigational and thus the drug, device or biological product may be assessed only on the basis of medical necessity. Pharmacy Coverage Guidelines are subject to change as new information becomes available. For purposes of this Pharmacy Coverage Guideline, the terms "experimental" and "investigational" are considered to be interchangeable. BLUE CROSS®, BLUE SHIELD® and the Cross and Shield Symbols are registered service marks of the Blue Cross and Blue Shield Association, an association of independent Blue Cross and Blue Shield Plans. -

Supplementary Information
Supplementary Information Network-based Drug Repurposing for Novel Coronavirus 2019-nCoV Yadi Zhou1,#, Yuan Hou1,#, Jiayu Shen1, Yin Huang1, William Martin1, Feixiong Cheng1-3,* 1Genomic Medicine Institute, Lerner Research Institute, Cleveland Clinic, Cleveland, OH 44195, USA 2Department of Molecular Medicine, Cleveland Clinic Lerner College of Medicine, Case Western Reserve University, Cleveland, OH 44195, USA 3Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH 44106, USA #Equal contribution *Correspondence to: Feixiong Cheng, PhD Lerner Research Institute Cleveland Clinic Tel: +1-216-444-7654; Fax: +1-216-636-0009 Email: [email protected] Supplementary Table S1. Genome information of 15 coronaviruses used for phylogenetic analyses. Supplementary Table S2. Protein sequence identities across 5 protein regions in 15 coronaviruses. Supplementary Table S3. HCoV-associated host proteins with references. Supplementary Table S4. Repurposable drugs predicted by network-based approaches. Supplementary Table S5. Network proximity results for 2,938 drugs against pan-human coronavirus (CoV) and individual CoVs. Supplementary Table S6. Network-predicted drug combinations for all the drug pairs from the top 16 high-confidence repurposable drugs. 1 Supplementary Table S1. Genome information of 15 coronaviruses used for phylogenetic analyses. GenBank ID Coronavirus Identity % Host Location discovered MN908947 2019-nCoV[Wuhan-Hu-1] 100 Human China MN938384 2019-nCoV[HKU-SZ-002a] 99.99 Human China MN975262 -
![Selective Labeling of Serotonin Receptors Byd-[3H]Lysergic Acid](https://docslib.b-cdn.net/cover/9764/selective-labeling-of-serotonin-receptors-byd-3h-lysergic-acid-319764.webp)
Selective Labeling of Serotonin Receptors Byd-[3H]Lysergic Acid
Proc. Nati. Acad. Sci. USA Vol. 75, No. 12, pp. 5783-5787, December 1978 Biochemistry Selective labeling of serotonin receptors by d-[3H]lysergic acid diethylamide in calf caudate (ergots/hallucinogens/tryptamines/norepinephrine/dopamine) PATRICIA M. WHITAKER AND PHILIP SEEMAN* Department of Pharmacology, University of Toronto, Toronto, Canada M5S 1A8 Communicated by Philip Siekevltz, August 18,1978 ABSTRACT Since it was known that d-lysergic acid di- The objective in this present study was to improve the se- ethylamide (LSD) affected catecholaminergic as well as sero- lectivity of [3H]LSD for serotonin receptors, concomitantly toninergic neurons, the objective in this study was to enhance using other drugs to block a-adrenergic and dopamine receptors the selectivity of [3HJISD binding to serotonin receptors in vitro by using crude homogenates of calf caudate. In the presence of (cf. refs. 36-38). We then compared the potencies of various a combination of 50 nM each of phentolamine (adde to pre- drugs on this selective [3H]LSD binding and compared these clude the binding of [3HJLSD to a-adrenoceptors), apmo ie, data to those for the high-affinity binding of [3H]serotonin and spiperone (added to preclude the binding of [3H[LSD to (39). dopamine receptors), it was found by Scatchard analysis that the total number of 3H sites went down to 300 fmol/mg, compared to 1100 fmol/mg in the absence of the catechol- METHODS amine-blocking drugs. The IC50 values (concentrations to inhibit Preparation of Membranes. Calf brains were obtained fresh binding by 50%) for various drugs were tested on the binding of [3HLSD in the presence of 50 nM each of apomorphine (A), from the Canada Packers Hunisett plant (Toronto). -

Antibiotic Practice Change to Curtail Linezolid Use in Pediatric Hospitalized Patients in Hawai‘I with Uncomplicated Skin and Soft Tissue Infections
Antibiotic Practice Change to Curtail Linezolid Use in Pediatric Hospitalized Patients in Hawai‘i with Uncomplicated Skin and Soft Tissue Infections Cheryl Okado MD and Tori Teramae BS Abstract MRSA coverage, clindamycin became a widely-used antibi- otic for treating uncomplicated SSTIs in children. Following Antimicrobial resistance affects health care providers’ choice of antibiotics increasing clindamycin use, increasing clindamycin resistance in the treatment of skin and soft tissue infections (SSTIs). Based on local was soon noted, particularly in MRSA isolates.2 Prior to 2010, antibiotic susceptibility data showing high clindamycin resistance and high MRSA predominance appeared to peak, and since then it has MRSA prevalence, a change in antibiotic regimen for children hospitalized for 3 uncomplicated SSTIs was instituted in an attempt to curb the use of linezolid. been decreasing. The prevalence of clindamycin-resistant GAS A retrospective chart review was performed on 278 pediatric patients with has been known to vary with time and location with US rates uncomplicated SSTIs hospitalized at Kapi‘olani Medical Center for Women ranging from 4%-41% since the early 2000s.4 and Children in Hawai‘i from May 2014 to April 2015 and November 2015 to October 2016. Data consisted of 12 months of baseline data and 12 In Hawai‘i, methicillin and clindamycin resistance patterns of months of data post-implementation of an antibiotic combination regimen of SA initially followed similar increasing trends. Antibiograms 2 widely-used antibiotics: high-dose cefazolin and high-dose clindamycin. at Hawai‘i’s children’s hospital, Kapi‘olani Medical Center for Practitioners were encouraged to use cefazolin alone if clinical suspicion was high for single-organism infection with group A streptococcus. -

Ergot Alkaloids As Dopamine Agonists: Comparison in Two Rodent Models
European Journal of Pharmacology, 37 (1976) 295-302 295 © North-Holland Publishing Company, Amsterdam - Printed in The Netherlands ERGOT ALKALOIDS AS DOPAMINE AGONISTS: COMPARISON IN TWO RODENT MODELS GILL ANLEZARK, CHRIS PYCOCK and BRIAN MELDRUM Department of Neurology, Institute of Psychiatry, Denmark Hill, London, SE5 8AF, U.K. Received 18 December 1975, revised MS received 20 February 1976, accepted 26 February 1976 G. ANLEZARK, C. PYCOCK and B. MELDRUM, Ergot alkaloids as dopamine agonists: comparison in two rodent models, European J. Pharmacol. 37 (1976) 295-302. A series of ergot alkaloids, together with the DA agonists apomorphine and piribedil, were tested for protec- tive effects against audiogenic seizures in an inbred strain of mice (DBA/2) and for induction of circling behaviour in mice with unilateral destruction of one nigrostriatal DA pathway. The order of potency against audiogenic sei- zures was apomorphine> ergocornine> bromocryptine > ergometrine> LSD> methysergide > piribedil while that observed in the rotating mouse model was apomorphine> ergometrine> ergocornine> brornocryptine > piribedil. LSD caused only weak circling behaviour even when administered in high doses (> 1 mg/kg). Methyser- gide was ineffective. Prior administration of the neuroleptic agent haloperidol blocked the effect of DA agonists and of ergot alkaloids in both animal models. The possible action of ergot alkaloids as DA agonists is discussed. Ergot alkaloids Audiogenic seizures Dopamine agonists Circling behaviour 1. Introduction gic synapses, in two rodent pharmacological models. The first model studied is 'audiogen- The pharmacology of the ergot alkaloids is ic' seizures in genetically susceptible mice. complex and not well understood. Peripheral- The severity of the seizure responses to audi- ly, they act on smooth muscle as 5-hydroxy- tory stimulation can be modified by a variety tryptamine (5-HT) antagonists (Goodman and of drugs believed to act on monoaminergic Gilman, 1971) and as a-adrenergic blockers transmission in the brain (Lehmann, 1970). -

Male Anorgasmia: from “No” to “Go!”
Male Anorgasmia: From “No” to “Go!” Alexander W. Pastuszak, MD, PhD Assistant Professor Center for Reproductive Medicine Division of Male Reproductive Medicine and Surgery Scott Department of Urology Baylor College of Medicine Disclosures • Endo – speaker, consultant, advisor • Boston Scientific / AMS – consultant • Woven Health – founder, CMO Objectives • Understand what delayed ejaculation (DE) and anorgasmia are • Review the anatomy and physiology relevant to these conditions • Review what is known about the causes of DE and anorgasmia • Discuss management of DE and anorgasmia Definitions Delayed Ejaculation (DE) / Anorgasmia • The persistent or recurrent delay, difficulty, or absence of orgasm after sufficient sexual stimulation that causes personal distress Intravaginal Ejaculatory Latency Time (IELT) • Normal (median) à 5.4 minutes (0.55-44.1 minutes) • DE à mean IELT + 2 SD = 25 minutes • Incidence à 2-11% • Depends in part on definition used J Sex Med. 2005; 2: 492. Int J Impot Res. 2012; 24: 131. Ejaculation • Separate event from erection! • Thus, can occur in the ABSENCE of erection! Periurethral muscle Sensory input - glans (S2-4) contraction Emission Vas deferens contraction Sympathetic input (T12-L1) SV, prostate contraction Bladder neck contraction Expulsion Bulbocavernosus / Somatic input (S1-3) spongiosus contraction Projectile ejaculation J Sex Med. 2011; 8 (Suppl 4): 310. Neurochemistry Sexual Response Areas of the Brain • Pons • Nucleus paragigantocellularis Neurochemicals • Norepinephrine, serotonin: • Inhibit libido, -

Biotechnology INITIATION REPORT Member FINRA/SIPC
INSTITUTIONAL RESEARCH Biotechnology INITIATION REPORT Member FINRA/SIPC Toll-Free: 561-391-5555 ⬧ www.DawsonJames.com ⬧ 1 North Federal Highway - Suite 500 ⬧ Boca Raton, FL 33432 Jaguar (NASDAQ/JAGX) January 3, 2019 BUY It’s Time for a Natural Solution Jason H. Kolbert Head of Healthcare Research 646-465-6891 It's time for a natural solution. Crofelemer, brand name Mytesi, is a natural product derived from an abundant source, the Corton lechleri tree. It treats diarrhea at its [email protected] source by normalizing secretion of water into the gut. Mytesi is the only FDA approved antisecretory diarrhea treatment on the market today. Investment Highlights Current Price $0.28 Mytesi revenues are starting to build, reflecting Jaguar's focused product launch Price Target $1.00 efforts. Chronic diarrhea is a common side-effect of HIV therapy and annually Estimates F2017A F2018E F2019E impacts greater than 250,000 people in the U.S. Jaguar is looking to address this Revenues ($000s) $ 4,361 $ 4,620 $ 16,054 population with a new salesforce that is now actively promoting the drug. Sales growth 1Q March $ 822 $ 804 $ 2,482 since promotion efforts began in 2017 looks favorable. We estimate Mytesi could 2Q June $ 897 $ 884 $ 3,085 3Q September $ 1,100 $ 1,132 $ 4,542 reach $4.4M in revenues this year, $15.7M in 2019, and $28.7M in 2020, driving the 4Q December $ 1,542 $ 1,800 $ 5,946 company to break-even. We estimate that by 2028 Mytesi in HIV alone can reach F2017A F2018E F2019E $76M in revenues, which would represent less than 10% of the total HIV market. -

Hallucinogens: an Update
National Institute on Drug Abuse RESEARCH MONOGRAPH SERIES Hallucinogens: An Update 146 U.S. Department of Health and Human Services • Public Health Service • National Institutes of Health Hallucinogens: An Update Editors: Geraline C. Lin, Ph.D. National Institute on Drug Abuse Richard A. Glennon, Ph.D. Virginia Commonwealth University NIDA Research Monograph 146 1994 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service National Institutes of Health National Institute on Drug Abuse 5600 Fishers Lane Rockville, MD 20857 ACKNOWLEDGEMENT This monograph is based on the papers from a technical review on “Hallucinogens: An Update” held on July 13-14, 1992. The review meeting was sponsored by the National Institute on Drug Abuse. COPYRIGHT STATUS The National Institute on Drug Abuse has obtained permission from the copyright holders to reproduce certain previously published material as noted in the text. Further reproduction of this copyrighted material is permitted only as part of a reprinting of the entire publication or chapter. For any other use, the copyright holder’s permission is required. All other material in this volume except quoted passages from copyrighted sources is in the public domain and may be used or reproduced without permission from the Institute or the authors. Citation of the source is appreciated. Opinions expressed in this volume are those of the authors and do not necessarily reflect the opinions or official policy of the National Institute on Drug Abuse or any other part of the U.S. Department of Health and Human Services. The U.S. Government does not endorse or favor any specific commercial product or company. -
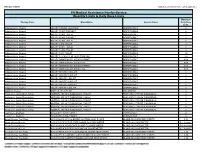
Quantity Limits/Daily Dose Limits
Effective 07/20/21 Alphabetical by Brand Name (when applicable) PA Medical Assistance Fee-for-Service Quantity Limits & Daily Dose Limits Maximum Therapy Class Brand Name Generic Name Daily Dose Limit Antipsychotics, Atypical ABILIFY 1 MG/ML SOLUTION ARIPIPRAZOLE 25 Antipsychotics, Atypical ABILIFY 10 MG DISCMELT ARIPIPRAZOLE 2 Antipsychotics, Atypical ABILIFY 10 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 15 MG DISCMELT ARIPIPRAZOLE 2 Antipsychotics, Atypical ABILIFY 15 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 2 MG TABLET ARIPIPRAZOLE 2 Antipsychotics, Atypical ABILIFY 20 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 30 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 5 MG TABLET ARIPIPRAZOLE 1.5 Antipsychotics, Atypical ABILIFY 9.75 MG/1.3 ML INJECTION VIAL ARIPIPRAZOLE 3.9 Antipsychotics, Atypical ABILIFY MAINTENA ER 300 MG SYRINGE ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MAINTENA ER 300 MG VIAL ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MAINTENA ER 400 MG SYRINGE ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MAINTENA ER 400 MG VIAL ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MYCITE 10 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 15 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 2 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 20 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 30 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 5 MG KIT ARIPIPRAZOLE 1 Antivirals, Herpes ABREVA 10% -

Drug News Nov Issue 25
D r u g N e w s 藥 物 情 報 Issue No. 25 : November 2011 This is a monthly digest of local and overseas drug safety news and information released in the previous month. For the latest news and information, please refer to public announcements or the website of the Drug Office of the Department of Health (http://www.drugoffice.gov.hk). Safety Update US: Updated information about drug Canada and FDA released similar alerts on the interaction of Zyvox (linezolid) and interaction between methylene blue and serotonin Methylene Blue with serotonergic reuptake inhibitors. At the same time, FDA also released alert on the interaction between linezolid psychiatric medications and serotonin reuptake inhibitors. The news had 20 October 2011 – The Food and Drug been reported in Issue No. 17 and 22 of Drug News Administration (FDA) provided additional respectively. In addition, the Department of Health information about the possible serotonin syndrome (DH) issued press statement on 18 February 2011 in patients taking serotonergic psychiatric and letters were also sent to healthcare professionals medications and linezolid/ methylene blue. It was in February 2011 and July 2011. found that not all serotonergic psychiatric drugs had The product certificate holders of the an equal capacity to cause serotonin syndrome with pharmaceutical products containing methylene blue, use of linezolid/ methylene blue. According to the linezolid and serotonergic psychiatric medications FDA's Adverse Event Reporting System (AERS), have been requested to update the sales pack and/or most cases of serotonin syndrome with linezolid or package insert of these products to include methylene blue occurred in patients taking specific information about the potential drug interactions.