Site Characteristics of Leitneria Floridana (Leitneriaceae) As Related to Potential Biological Control of the Invasive Tree-Of-Heaven, Ailanthus Altissima
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Department of Planning and Zoning
Department of Planning and Zoning Subject: Howard County Landscape Manual Updates: Recommended Street Tree List (Appendix B) and Recommended Plant List (Appendix C) - Effective July 1, 2010 To: DLD Review Staff Homebuilders Committee From: Kent Sheubrooks, Acting Chief Division of Land Development Date: July 1, 2010 Purpose: The purpose of this policy memorandum is to update the Recommended Plant Lists presently contained in the Landscape Manual. The plant lists were created for the first edition of the Manual in 1993 before information was available about invasive qualities of certain recommended plants contained in those lists (Norway Maple, Bradford Pear, etc.). Additionally, diseases and pests have made some other plants undesirable (Ash, Austrian Pine, etc.). The Howard County General Plan 2000 and subsequent environmental and community planning publications such as the Route 1 and Route 40 Manuals and the Green Neighborhood Design Guidelines have promoted the desirability of using native plants in landscape plantings. Therefore, this policy seeks to update the Recommended Plant Lists by identifying invasive plant species and disease or pest ridden plants for their removal and prohibition from further planting in Howard County and to add other available native plants which have desirable characteristics for street tree or general landscape use for inclusion on the Recommended Plant Lists. Please note that a comprehensive review of the street tree and landscape tree lists were conducted for the purpose of this update, however, only -

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -
Checklist of Illinois Native Trees
Technical Forestry Bulletin · NRES-102 Checklist of Illinois Native Trees Jay C. Hayek, Extension Forestry Specialist Department of Natural Resources & Environmental Sciences Updated May 2019 This Technical Forestry Bulletin serves as a checklist of Tree species prevalence (Table 2), or commonness, and Illinois native trees, both angiosperms (hardwoods) and gym- county distribution generally follows Iverson et al. (1989) and nosperms (conifers). Nearly every species listed in the fol- Mohlenbrock (2002). Additional sources of data with respect lowing tables† attains tree-sized stature, which is generally to species prevalence and county distribution include Mohlen- defined as having a(i) single stem with a trunk diameter brock and Ladd (1978), INHS (2011), and USDA’s The Plant Da- greater than or equal to 3 inches, measured at 4.5 feet above tabase (2012). ground level, (ii) well-defined crown of foliage, and(iii) total vertical height greater than or equal to 13 feet (Little 1979). Table 2. Species prevalence (Source: Iverson et al. 1989). Based on currently accepted nomenclature and excluding most minor varieties and all nothospecies, or hybrids, there Common — widely distributed with high abundance. are approximately 184± known native trees and tree-sized Occasional — common in localized patches. shrubs found in Illinois (Table 1). Uncommon — localized distribution or sparse. Rare — rarely found and sparse. Nomenclature used throughout this bulletin follows the Integrated Taxonomic Information System —the ITIS data- Basic highlights of this tree checklist include the listing of 29 base utilizes real-time access to the most current and accept- native hawthorns (Crataegus), 21 native oaks (Quercus), 11 ed taxonomy based on scientific consensus. -

Oaks for Nebraska Justin R
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Nebraska Statewide Arboretum Publications Nebraska Statewide Arboretum 2013 Oaks for Nebraska Justin R. Evertson University of Nebraska - Lincoln, [email protected] Follow this and additional works at: http://digitalcommons.unl.edu/arboretumpubs Part of the Botany Commons, and the Forest Biology Commons Evertson, Justin R., "Oaks for Nebraska" (2013). Nebraska Statewide Arboretum Publications. 1. http://digitalcommons.unl.edu/arboretumpubs/1 This Article is brought to you for free and open access by the Nebraska Statewide Arboretum at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Nebraska Statewide Arboretum Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Oaks for Nebraska Justin Evertson, Nebraska Statewide Arboretum arboretum.unl.edu or retreenebraska.unl.edu R = belongs to red oak group—acorns mature over two seasons & leaves typically have pointed lobes. W = belongs to white oak group— acorns mature in one season & leaves typically have rounded lobes. Estimated size range is height x spread for trees growing in eastern Nebraska. A few places to see oaks: Indian Dwarf chinkapin oak, Quercus Cave State Park; Krumme Arboretum Blackjack oak, Quercus prinoides (W) in Falls City; Peru State College; marilandica (R) Variable habit from shrubby to Fontenelle Nature Center in Bellevue; Shorter and slower growing than tree form; prolific acorn producer; Elmwood Park in Omaha; Wayne most oaks with distinctive tri- can have nice yellow fall color; Park in Waverly; University of lobed leaves; can take on a very national champion grows near Nebraska Lincoln; Lincoln Regional natural look with age; tough and Salem Nebraska; 10-25’x 10-20’. -

Illinois Native Plant Society 2019 Plant List Herbaceous Plants
Illinois Native Plant Society 2019 Plant List Plant Sale: Saturday, May 11, 9:00am – 1:00pm Illinois State Fairgrounds Commodity Pavilion (Across from Grandstand) Herbaceous Plants Scientific Name Common Name Description Growing Conditions Comment Dry to moist, Sun to part Blooms mid-late summer Butterfly, bee. Agastache foeniculum Anise Hyssop 2-4', Lavender to purple flowers shade AKA Blue Giant Hyssop Allium cernuum var. Moist to dry, Sun to part Nodding Onion 12-18", Showy white flowers Blooms mid summer Bee. Mammals avoid cernuum shade 18", White flowers after leaves die Allium tricoccum Ramp (Wild Leek) Moist, Shade Blooms summer Bee. Edible back Moist to dry, Part shade to Blooms late spring-early summer Aquilegia canadensis Red Columbine 30", Scarlet and yellow flowers shade Hummingbird, bee Moist to wet, Part to full Blooms late spring-early summer Showy red Arisaema dracontium Green Dragon 1-3', Narrow greenish spadix shade fruits. Mammals avoid 1-2', Green-purple spadix, striped Moist to wet, Part to full Blooms mid-late spring Showy red fruits. Arisaema triphyllum Jack-in-the-Pulpit inside shade Mammals avoid Wet to moist, Part shade to Blooms late spring-early summer Bee. AKA Aruncus dioicus Goatsbeard 2-4', White fluffy panicles in spring shade Brides Feathers Wet to moist, Light to full Blooms mid-late spring Mammals avoid. Asarum canadense Canadian Wildginger 6-12", Purplish brown flowers shade Attractive groundcover 3-5', White flowers with Moist, Dappled sun to part Blooms summer Monarch larval food. Bee, Asclepias exaltata Poke Milkweed purple/green tint shade butterfly. Uncommon Blooms mid-late summer Monarch larval Asclepias hirtella (Tall) Green Milkweed To 3', Showy white flowers Dry to moist, Sun food. -

Beneficial Trees for Wildlife Forestry and Plant Materials Technical Note
United States Department of Agriculture Natural Resources Conservation Service Technical Note No: TX-PM-16-01 August 2016 Beneficial Trees for Wildlife Forestry and Plant Materials Technical Note Background Trees provide shelter and food sources for a wide array of wildlife. White tail deer browse leaves and twigs along with acorns each fall and winter when other food sources are unavailable. More than 100 animal species eat acorns including rabbits, squirrels, wild hog, and gamebirds (Ober 2014). Songbirds and small mammals consume fruits and seeds. Wood peckers (Melanerpes sp.) and red tailed hawk (Buteo jamaicencis) nest in the cavities of hollow or dead trees (Dickson and Connor 1982). Butterflies, moths, and honeybees use trees as larval hosts, nectar sources, and shelter (Hill and Webster 1995). At right is a map illustrating forest types within the Western Gulf Coastal Plain. The Western Gulf Coastal Plain has a diversity of native hardwoods along with three species of southern pines (longleaf (Pinus palustris), shortleaf (Pinus echinata) and loblolly (Pinus taeda). Important native hardwoods used commercially and for wildlife include mockernut hickory (Carya tomentosa), hackberry (Celtis laevigata), green ash (Fraxinus pennsylvanica), black walnut (Juglans nigra), sweetgum (Liquidambar styraciflua), black tupelo (Nyssa sylvatica), white oak (Quercus alba), southern red oak (Quercus falcata), water oak (Quercus nigra), willow oak (Quercus phellos), shumard oak (Quercus shumardii), post oak (Quercus stellata), bald cypress (Taxodium distichum), and American elm (Ulmus americana) (Diggs 2006). 1 Purpose The purpose of this technical note is to assist conservation planners and land managers by providing basic tree establishment information and a list of beneficial wildlife trees (Table 1) when they are planning wildlife and pollinator habitat in east Texas, western Louisiana, southwestern Arkansas, and southeastern Oklahoma. -

Quercus ×Coutinhoi Samp. Discovered in Australia Charlie Buttigieg
XXX International Oaks The Journal of the International Oak Society …the hybrid oak that time forgot, oak-rod baskets, pros and cons of grafting… Issue No. 25/ 2014 / ISSN 1941-2061 1 International Oaks The Journal of the International Oak Society … the hybrid oak that time forgot, oak-rod baskets, pros and cons of grafting… Issue No. 25/ 2014 / ISSN 1941-2061 International Oak Society Officers and Board of Directors 2012-2015 Officers President Béatrice Chassé (France) Vice-President Charles Snyers d’Attenhoven (Belgium) Secretary Gert Fortgens (The Netherlands) Treasurer James E. Hitz (USA) Board of Directors Editorial Committee Membership Director Chairman Emily Griswold (USA) Béatrice Chassé Tour Director Members Shaun Haddock (France) Roderick Cameron International Oaks Allen Coombes Editor Béatrice Chassé Shaun Haddock Co-Editor Allen Coombes (Mexico) Eike Jablonski (Luxemburg) Oak News & Notes Ryan Russell Editor Ryan Russell (USA) Charles Snyers d’Attenhoven International Editor Roderick Cameron (Uruguay) Website Administrator Charles Snyers d’Attenhoven For contributions to International Oaks contact Béatrice Chassé [email protected] or [email protected] 0033553621353 Les Pouyouleix 24800 St.-Jory-de-Chalais France Author’s guidelines for submissions can be found at http://www.internationaloaksociety.org/content/author-guidelines-journal-ios © 2014 International Oak Society Text, figures, and photographs © of individual authors and photographers. Graphic design: Marie-Paule Thuaud / www.lecentrecreatifducoin.com Photos. Cover: Charles Snyers d’Attenhoven (Quercus macrocalyx Hickel & A. Camus); p. 6: Charles Snyers d’Attenhoven (Q. oxyodon Miq.); p. 7: Béatrice Chassé (Q. acerifolia (E.J. Palmer) Stoynoff & W. J. Hess); p. 9: Eike Jablonski (Q. ithaburensis subsp. -
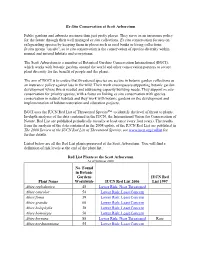
IUCN Red List of Threatened Species™ to Identify the Level of Threat to Plants
Ex-Situ Conservation at Scott Arboretum Public gardens and arboreta are more than just pretty places. They serve as an insurance policy for the future through their well managed ex situ collections. Ex situ conservation focuses on safeguarding species by keeping them in places such as seed banks or living collections. In situ means "on site", so in situ conservation is the conservation of species diversity within normal and natural habitats and ecosystems. The Scott Arboretum is a member of Botanical Gardens Conservation International (BGCI), which works with botanic gardens around the world and other conservation partners to secure plant diversity for the benefit of people and the planet. The aim of BGCI is to ensure that threatened species are secure in botanic garden collections as an insurance policy against loss in the wild. Their work encompasses supporting botanic garden development where this is needed and addressing capacity building needs. They support ex situ conservation for priority species, with a focus on linking ex situ conservation with species conservation in natural habitats and they work with botanic gardens on the development and implementation of habitat restoration and education projects. BGCI uses the IUCN Red List of Threatened Species™ to identify the level of threat to plants. In-depth analyses of the data contained in the IUCN, the International Union for Conservation of Nature, Red List are published periodically (usually at least once every four years). The results from the analysis of the data contained in the 2008 update of the IUCN Red List are published in The 2008 Review of the IUCN Red List of Threatened Species; see www.iucn.org/redlist for further details. -

University of Tennessee Instructor Copy
Copy This chapter is an edited version An Overview of the Physical of a manuscript by the same title Environment, Flora, and written by Edward W. Chester. Vegetation of Tennessee In addition to editing, some 2 material has been deleted from and some added to the original Instructormanuscript by the editors of this publication. Portions of the original manuscript were condensed from Guide to the Vascular Plants of Tennessee, compiled and edited by the Tennessee Flora Committee. Copyright © 2015 by The University of Tennessee Press, Knoxville. Used by permission granted to Edward W. Chester. See author’s notes for additional Tennessee information. of University Copy AUTHOR'S NOTES A more complete discussion of the topics in this chapter can be found in Chapter 1, “The Physical Environment of Tennessee” (written by Edward W. Chester) and Chapter 3, ”An Overview of the Vegetation of Tennessee” (coauthored by Hal R. DeSelm and William H. Martin) in the Guide to the Vascular Plants of Tennessee referenced at the beginning of this chapter. The contents of the two chapters are based on almost 200 combined years of study by the three authors and the nearly 100 references they cite. The authors are: Instructor Edward W. Chester, Professor Emeritus of Biology and Botany, Austin Peay State University, Clarksville, Tenn. Hal R. DeSelm (deceased), Professor Emeritus of Botany and the Graduate Program in Ecology, University of Tennessee at Knoxville. Dr. DeSelm died on July 12, 2011. William H. Martin, Professor Emeritus of Biology and Director of the Division of Natural Areas, Eastern Kentucky University, Richmond. He served as Commissioner of Kentucky's Department for Natural Resources from 1992 to 1998. -

First Phylogeny of Bitterbush Family, Picramniaceae (Picramniales)
plants Article First Phylogeny of Bitterbush Family, Picramniaceae (Picramniales) Alexey Shipunov 1,*, Shyla Carr 1, Spencer Furniss 1, Kyle Pay 1 and José Rubens Pirani 2 1 Minot State University, Minot, ND 58707, USA; [email protected] (S.C.); [email protected] (S.F.); [email protected] (K.P.) 2 University of São Paulo, São Paulo 01000-000, Brazil; [email protected] * Correspondence: [email protected] Received: 17 December 2019; Accepted: 19 February 2020; Published: 21 February 2020 Abstract: Picramniaceae is the only member of Picramniales which is sister to the clade (Sapindales (Huerteales (Malvales, Brassicales))) in the rosidsmalvids. Not much is known about most aspects of their ecology, geography, and morphology. The family is restricted to American tropics. Picramniaceae representatives are rich in secondary metabolites; some species are known to be important for pharmaceutical purposes. Traditionally, Picramniaceae was classified as a subfamily of Simaroubaceae, but from 1995 on, it has been segregated containing two genera, Picramnia and Alvaradoa, with the recent addition of a third genus, Nothotalisia, described in 2011. Only a few species of the family have been the subject of DNA-related research, and fewer than half of the species have been included in morphological phylogenetic analyses. It is clear that Picramniaceae remains a largely under-researched plant group. Here we present the first molecular phylogenetic tree of the group, based on both chloroplast and nuclear markers, widely adopted in the plant DNA barcoding. The main findings are: The family and its genera are monophyletic and Picramnia is sister to two other genera; some clades corroborate previous assumptions of relationships made on a morphological or geographical basis, while most parts of the molecular topology suggest high levels of homoplasy in the morphological evolution of Picramnia. -

Bbyct-133 Plant Ecology and Taxonomy
BBYCT-133 PLANT ECOLOGY AND Indira Gandhi TAXONOMY National Open University School of Sciences VOL 2 PLANT ECOLOGY AND TAXONOMY BLOCK 3 PLANT TAXONOMY - TOOLS AND EVIDENCES 5 BLOCK 4 NOMENCLATURE AND SYSTEMS OF CLASSIFICATION 105 One of the greatest assets of a sound classification is its predictive value. Mayr (1969) PLANT ECOLOGY AND TAXONOMY In this volume 2 you are going to study about plant taxonomy. Taxonomy is the most relevant field of enquiry for modern man. Plant taxonomy is a 2 credit course which comprises of Block 3 and Block 4.Both of these blocks consist of five units each. Plant taxonomy is a fundamental science - the science of classifying plants into groups and identifying them. The progress of civilization marked a relative increase in man’s knowledge regarding plants - their identification, naming and classification on the basis of his needs. During last three centuries, plant taxonomy has developed from a completely morphology oriented static and descriptive discipline to a most dynamic area of study. The first classification of plants was thus according to their usefulness or not. When man came to realize that most of the plants are of some or other use, he classified them into herbs, shrubs and trees. This was followed by various “Natural “systems of classification. Taxonomy is related to morphology, anatomy, embryology, cytology and chemistry. In this course we have tried to establish the relationship of taxonomy with all the above given branches. Over the last few decades, the availability and usage of comparative and recombinant technology data from different species has made taxonomy more authentic and complete. -

Wolf River Greenway Level 1 Arboretum
Wolf River Greenway Level 1 Arboretum Map Created by: W. Ryan Hall, Wolf River Conservancy d & R Shelby County 4-H ve August, 2014 _ ro G t u n al W 19 18 W O 17 20 L 21 F 22 R I H V u m 24 23 E p 16 R h 25 r e 26 y s 27 B 28 lv d 29 15 30 31 14 33 32 13 12 11 9 8 7 6 5 34 4 H u m 35 p h re y s B 36 lv d 3 !i 2 ® 1 37 0 175 350 700 Feet 38 1 inch = 350 feet Wolf River Greenway Arboretum Number Common Name Scientific Name Family Name 1 Eastern Red Cedar Juniperus virginiana Cupressaceae - Cypress Family 2 Black Cherry Prunus serotina Rosaceae - Rose Family 3 Silver Maple Acer saccharinum Aceraceae - Maple Family 4 Green Ash Fraxinus pensylvanica Oleaceae - Olive Family 5 Sugarberry Celtis laevigata Ulmaceae - Elm Family 6 Sycamore Platanus occidentalis Platanaceae - Sycamore Family 7 Slippery Elm Ulmus rubra Ulmaceae - Elm Family 8 Boxelder Acer negundo Aceraceae - Maple Family 9 Baldcypress Taxodium distichum Taxodiaceae - Baldcypress Family 10 Flowering Dogwood Cornus florida Cornaceae - Dogwood Family 11 Yellow-poplar Liriodendron tulipifera Magnoliaceae - Magnolia Family 12 Common Pawpaw Asimina triloba Annonaceae - Custard-Apple Family 13 River Birch Betula nigra Betulaceae - Birch Family 14 Red Mulberry Morus rubra Moraceae - Mulberry Family 15 Devils Walking Stick Aralia spinosa Araliaceae - Ivy Family 16 Black Tupelo Nyssa sylvatica Nyssaceae - Tupelo Family 17 American Sweetgum Liquidambar styraciflua Hamamelidaceae - Witchhazel Family 18 Black Locust Robinia pseudoacacia Fabaceae - Legume Family 19 Japanese Zelkova Zelkova serrata