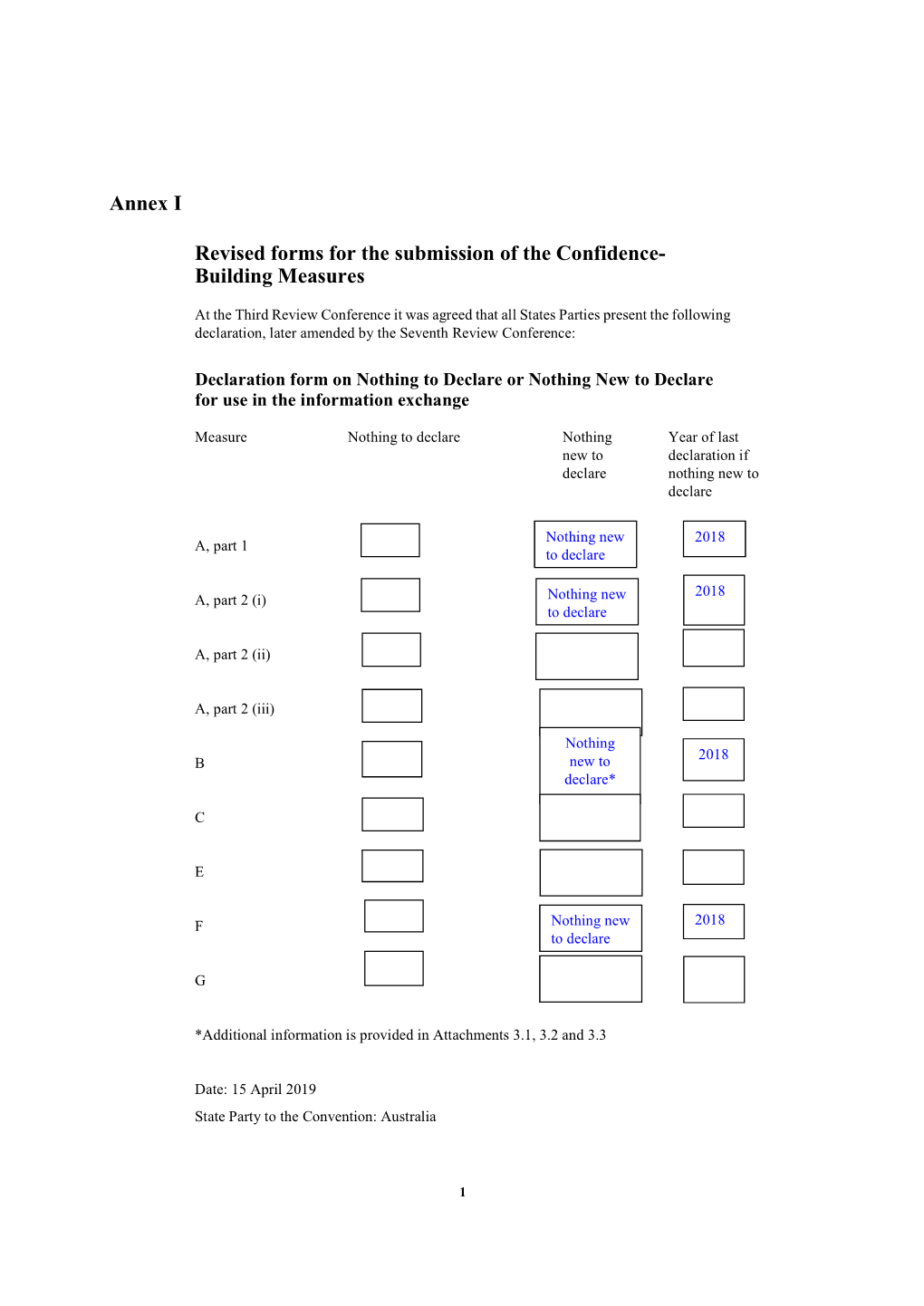
Annex I Revised Forms for the Submission of the Confidence
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Final Report
Final Report Coordination of Banana Industry R&D (Panama TR4) Project leader: Jim Pekin Delivery partner: Australian Banana Growers’ Council Project code: BA14012 Hort Innovation – Final Report Project: Coordination of Banana Industry R&D (Panama TR4) – BA14012 Disclaimer: Horticulture Innovation Australia Limited (Hort Innovation) makes no representations and expressly disclaims all warranties (to the extent permitted by law) about the accuracy, completeness, or currency of information in this Final Report. Users of this Final Report should take independent action to confirm any information in this Final Report before relying on that information in any way. Reliance on any information provided by Hort Innovation is entirely at your own risk. Hort Innovation is not responsible for, and will not be liable for, any loss, damage, claim, expense, cost (including legal costs) or other liability arising in any way (including from Hort Innovation or any other person’s negligence or otherwise) from your use or non‐use of the Final Report or from reliance on information contained in the Final Report or that Hort Innovation provides to you by any other means. Funding statement: This project has been funded by Hort Innovation, using the banana research and development levy and contributions from the Australian Government. Hort Innovation is the grower‐owned, not‐for‐profit research and development corporation for Australian horticulture. Publishing details: ISBN 978 0 7341 4433 1 Published and distributed by: Hort Innovation Level 8 1 Chifley Square -

APPS Newsletter Vol 27, No. 3 December 2014 in This Edition
APPS Newsletter Vol 27, No. 3 December 2014 In this edition: Page 2. President’s message Page 3. News from the Business Manager Page 4. New members Page 4. Dates for your Diary Page 5. Regional news from New South Wales Page 8. Regional news from Victoria Page 10. Regional news from Tasmania Page 11. Report on the 8th Australasian Soilborne Diseases Symposium Page 15. Report on the 11th Australasian Plant Virology Workshop APPS NEWS is the official newsletter of the Australasian Plant Pathology Society, published electronically 3 times per year. Items for inclusion should be sent to: Dr Will Cuddy, Plant Breeding Institute, University of Sydney, Private Mail Bag 4011, Narellan, NSW, 2567. Ph. 02 9351 8871, Email: [email protected] Next deadline: March 31 2015 Web Site: http://www.australasianplantpathologysociety.org.au/ 1 APPS December 2014 Vol 27 No. 3 President’s Message 2014 seems to have gone by very quickly. The Management Committee is busy preparing for the upcoming Annual General Meeting, to be held on Thursday 11 December 2014. By the time you receive this newsletter, the AGM will be over, so I hope you were able to take up the invitation to join the meeting and contribute to the running of our Society. Progress towards the goals outlined in the 2-year plan has been documented in the President’s report prepared for the AGM (see http://www.appsnet.org/members/General/AGM%202014/index.aspx). When you next visit the APPS website I hope you will appreciate the improvements implemented by the Business Manager, Peter Williamson, to make the website more user-friendly. -

Characterisation and Management of Fusarium Wilt of Watermelon
Characterisation and management of Fusarium wilt of watermelon Dr Lucy Tran-Nguyen Northern Territory Department of Primary Industry and Fisheries Project Number: VM12001 Authors: Lucy Tran-Nguyen Cassie McMaster 1 VM12001 Horticulture Innovation Australia Limited (HIA Ltd) and the Northern Territory Department of Primary Industry and Fisheries make no representations and expressly disclaim all warranties (to the extent permitted by law) about the accuracy, completeness, or currency of information in this Final Report. Users of this Final Report should take independent action to confirm any information in this Final Report before relying on that information in any way. Reliance on any information provided by HIA Ltd is entirely at your own risk. HIA Ltd is not responsible for, and will not be liable for, any loss, damage, claim, expense, cost (including legal costs) or other liability arising in any way (including from HIA Ltd or any other person’s negligence or otherwise) from your use or non-use of the Final Report or from reliance on information contained in the Final Report or that HIA Ltd provides to you by any other means. R&D projects: co-investment funding This project has been funded by Horticulture Innovation Australia Limited with co-investment from Monsanto Australia and Rijk Zwaan Australia Pty. Ltd and funds from the Australian Government. ISBN 978 0 7341 4359 4 Published and distributed by: Horticulture Innovation Australia Ltd Level 8 1 Chifley Square Sydney NSW 2000 Telephone: (02) 8295 2300 Fax: (02) 8295 2399 © Copyright -

Australian Bananas Magazine
Australian BananasIssue 40, Summer 2013-2014 Hopeful harvest Trial blocks yield answers in the search for disease-resistant varieties Page 10 Banana Page 20 Page 22 China- Freckle eradication Retail campaign Philippines study tour editorial & advertising AUSTRALIAN Rhyll Cronin 07 3278 4786 [email protected] art direction & design ToadShow Bananas Summer 2013-2014 2 Eton Street,Toowong contents 07 3335 4000 www.toadshow.com.au publisher Regulars Australian Banana 4 Chairman’s comment Growers’ Council Inc. 5 CEO’s comment ABN: 60 381 740 734 20 Marketing – Campaign puts a smile on chief executive officer retailers’ faces Jim Pekin 38 Health – Is rating food a five-star idea? research and 39 Membership – ABGC Juliane development manager 25 PAGE Henderson Jay Anderson 12 Industry news & MeeHua Wong office manager Alix Perry 6 Regional round up administration assistant 7 Banana grower AGM discusses industry Industry development sustainability Kaylar Packer 16 Lessons from the Philippines 8 Have your say on draft industry plan BOARD OF DIRECTORS Warnings for growers from a look at TR4 in the Philippines chairman Plant health Doug Phillips 22 Tour highlights threats, opportunities 9 Committee guides our six million dollar A group studies production in China and vice-chairman plan the Philippines Adrian Crema 28 PAGE The six-member team guiding the Banana 39 Robert Mayers in reef-grants role treasurer Plant Protection Program (BPPP) ABGC’s new Reef Water Quality Grants Paul Johnston Officer directors Features Marc Darveniza 10 Taking -

Market Access of Papua New Guinea Bananas (Musa Spp.) with Particular Respect to Banana Fly (Bactrocera Musae (Tryon)) (Diptera: Tephritidae)
Market Access of Papua New Guinea Bananas (Musa spp.) with Particular Respect to Banana Fly (Bactrocera musae (Tryon)) (Diptera: Tephritidae) Amanda Mararuai B.Sc Agriculture, Graduate Diploma in Applied Science A thesis submitted in fulfilment of the requirements for the degree of Doctor of Philosophy School of Natural Resource Sciences Queensland University of Technology Brisbane Australia April 2010 Keywords Bactrocera musae, banana fly, bananas, biosecurity, host availability, invasion biology, invasive, market access, Musa spp., novel environment, Papua New Guinea, pest risk analysis, population distribution ii Abstract International market access for fresh commodities is regulated by international accepted phytosanitary guidelines, the objectives of which are to reduce the biosecurity risk of plant pest and disease movement. Papua New Guinea (PNG) has identified banana as a potential export crop and to help meet international market access requirements, this thesis provides information for the development of a pest risk analysis (PRA) for PNG banana fruit. The PRA is a three step process which first identifies the pests associated with a particular commodity or pathway, then assesses the risk associated with those pests, and finally identifies risk management options for those pests if required. As the first step of the PRA process, I collated a definitive list on the organisms associated with the banana plant in PNG using formal literature, structured interviews with local experts, grey literature and unpublished file material held in PNG field research stations. I identified 112 organisms (invertebrates, vertebrate, pathogens and weeds) associated with banana in PNG, but only 14 of these were reported as commonly requiring management. For these 14 I present detailed information summaries on their known biology and pest impact. -

Banana Freckle (124)
Pacific Pests and Pathogens - Fact Sheets https://apps.lucidcentral.org/ppp/ Banana freckle (124) Photo 1. Small, raised spots with the flask-shaped fruiting bodies containing spores. The spots are at first reddish-brown later turning black and joining together Photo 2. Dark brown to black spots, 0.5-1 mm to form patches or streaks. diameter, on the upper leaf surface. Photo 3. Large numbers of spots on the leaves causing Photo 4. Early death of the leaf due to freckle infection them to turn yellow and die early. beginning at the leaf margin. Photo 5. Spots caused by the banana freckle fungus on the fruit. Common Name Banana freckle Scientific Name Previously, freckle was Phyllosticta musarum and its sexual form, Guignardia musae; now four species are recognised: Phyllosticta cavendishii, Phyllosticta musarum, Phyllosticta maculata, and Guignardia stevensii. Distribution Narrow. Asia, US (Hawaii), Oceania. Freckle was previously identified in the Pacific islands as Phyllosticta musarum (sexual state Guignardia musae). Under those names it was present in Fiji, Samoa, Tonga and Solomon Islands. From a recent (molecular and morphological) study Guignardia stevensii occurs only in Hawaii, and both Phyllosticta maculata and Phyllosticta cavendishii occur in the South Pacific, but their distribution is uncertain. It appears that Phyllosticta musarum now has a narrow distribution. Also, the study and subsequent research indicates that Phyllosticta maculata is present in Australia, Malaysia, Indonesia, Papua New Guinea, the Philippines and the South Pacific islands, and Phyllosticta cavendishii occurs in Australia (eradicated in 2019), East Timor, Hawaii, India, Indonesia, Malaysia, the Philippines, Sri Lanka, Taiwan, Vietnam, and in the Pacific islands (Tonga and Micronesia). -

Appendices Organisation Contact Details
Appendices Organisation contact details Organisation Website Phone Organisation Website Phone AgriFutures Australia agrifutures.com.au +61 2 6923 6900 Australian Macadamia Society australianmacadamias.org +61 2 6622 4933 Almond Board of Australia australianalmonds.com.au +61 8 8584 7053 Australian Mango Industry Association industry.mangoes.net.au +61 7 3278 3755 Apple and Pear Australia apal.org.au +61 3 9329 3511 Australian Melon Association melonsaustralia.org.au +61 413 101 646 Atlas of Living Australia ala.org.au +61 2 6218 3431 Australian National University anu.edu.au +61 2 6125 5111 Australasian Plant Pathology Society appsnet.org +61 7 4632 0467 Australian Olive Association australianolives.com.au +61 478 606 145 Australian Plant Biosecurity Science Australian Banana Growers’ Council abgc.org.au +61 7 3278 4786 apbsf.org.au Foundation Australian Blueberry Growers’ Association abga.com.au +61 490 092 273 Australian Processing Tomato Research aptrc.asn.au Australian Entomological Society austentsoc.org.au +61 3 9895 4462 Council ORGANISATION CONTACT DETAILS CONTACT ORGANISATION Australian Forest Products Association ausfpa.com.au +61 2 6285 3833 Australian Society for Microbiology theasm.org.au +61 1300 656 423 Australian Ginger Industry Association australianginger.org.au Australian Society of Agronomy agronomyaustralia.org Australian Government – Australian Centre aciar.gov.au +61 2 6217 0500 Australian Sweetpotato Growers aspg.com.au for International Agricultural Research APPENDIX 1: 1: APPENDIX Australian Table Grape Association -

The Biology of Musa L. (Banana)
The Biology of Musa L. (banana) [Photo credit: Janet Gorst, OGTR] Version 1: January 2008 This document provides an overview of baseline biological information relevant to risk assessment of genetically modified forms of the species that may be released into the Australian environment. For information on the Australian Government Office of the Gene Technology Regulator visit http://www.ogtr.gov.au [THIS PAGE HAS BEEN LEFT INTENTIONALLY BLANK ii The Biology of Musa L. (banana) Office of the Gene Technology Regulator TABLE OF CONTENTS PREAMBLE ...........................................................................................................................................1 SECTION 1 TAXONOMY .............................................................................................................1 SECTION 2 ORIGIN AND CULTIVATION ...............................................................................6 2.1 CENTRE OF DIVERSITY AND DOMESTICATION .............................................................. 6 2.2 COMMERCIAL USES...................................................................................................... 7 2.3 CULTIVATION IN AUSTRALIA....................................................................................... 9 2.3.1 Commercial propagation ...............................................................................................11 2.3.2 Scale of cultivation.........................................................................................................13 2.3.3 Cultivation -

Plant Biosecurity Timeline
PLANT BIOSECURITY IN AUSTRALIA A timeline of plant biosecurity events pre 1900s. Pre 1900s William Farrer joins the New For over 60,000 years, Aboriginal and Torres Strait Islander people undertook many agricultural and land management South Wales Department of practices, e.g. fire, while maintaining soil fertility. It is Agriculture with an aim to estimated they used around 5,000 species of food, including South Australia legislates the Vines Protection produce wheat varieties that will tubers such as yams, grain such as native millet, macadamia be rust-resistant, have a higher nuts, fruits and berries. In order to manage pests, they utilised Act 1874 and bans movement of vine cuttings protein content and better milling techniques such as the burning of leaves to repel insects and and rooted vines allowing them to remain free Vineyards in Geelong, fire to maintain species diversity in the landscape of the sap-sucking insects known as phylloxera Victoria are uprooted to and baking quality 1874 manage the outbreak of 1898 phylloxera Stem rust, a devastating wheat disease, 1882 forces farmers to grow wheat inland 1840 where it is drier and hotter 1860 1788 1845 1877 1884 1890 The first wheat is The first shipments of Phylloxera spreads Colonial governments sown in Australia Following the first reported north to New South Australian grown wheat are sighting of the insect in 1875, establish a series of The First Fleet Wales after it is exported to England phylloxera is positively annual national brings orange, detected in Camden identified in Australia in conferences to address lime and lemon 1839 Geelong, Victoria after how best to fight wheat seeds to New infested grapevine planting stem rust South Wales Prickly pear is introduced material is imported from into Australia for use as a Europe 1889 natural agricultural fence A severe rust epidemic costs the 1886 wheat industry £2-3 From 1886 to 1929, rabbit barrier million in lost 1859 and check fences are erected in production 13 European wild rabbits are released Queensland. -

Final Report
Final Report Australian Banana Industry Congress 2019 Project leader: Sonia Campbell Delivery partner: Australian Banana Growers’ Council (ABGC) Project code: BA17003 Hort Innovation – Final Report Project: Australian Banana Industry Congress 2019 BA17003 Disclaimer: Horticulture Innovation Australia Limited (Hort Innovation) makes no representations and expressly disclaims all warranties (to the extent permitted by law) about the accuracy, completeness, or currency of information in this Final Report. Users of this Final Report should take independent action to confirm any information in this Final Report before relying on that information in any way. Reliance on any information provided by Hort Innovation is entirely at your own risk. Hort Innovation is not responsible for, and will not be liable for, any loss, damage, claim, expense, cost (including legal costs) or other liability arising in any way (including from Hort Innovation or any other person’s negligence or otherwise) from your use or non-use of the Final Report or from reliance on information contained in the Final Report or that Hort Innovation provides to you by any other means. Funding statement: This project has been funded by Hort Innovation, using the banana industry research and development levy and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture. Publishing details: ISBN 978 0 7341 4567 3 Published and distributed by: Hort Innovation Level 7 141 Walker -

2017 National Plant Biosecurity Status Report
The National Plant Biosecurity Status Report 2017 © Plant Health Australia 2018 Disclaimer: This publication is published by Plant Health Australia for information purposes only. Information in the document is drawn from a variety of sources outside Plant Health This work is copyright. Apart from any use as Australia. Although reasonable care was taken in its preparation, Plant Health Australia does permitted under the Copyright Act 1968, no part not warrant the accuracy, reliability, completeness or currency of the information, or its may be reproduced by any process without prior usefulness in achieving any purpose. permission from Plant Health Australia. Given that there are continuous changes in trade patterns, pest distributions, control measures Requests and enquiries concerning reproduction and rights and agricultural practices, this report can only provide a snapshot in time. Therefore, all should be addressed to: information contained in this report has been collected for the 12 month period from 1 January 2017 to 31 December 2017, and should be validated and confirmed with the relevant Communications Manager organisations/authorities before being used. A list of contact details (including websites) is Plant Health Australia provided in the Appendices. 1/1 Phipps Close DEAKIN ACT 2600 To the fullest extent permitted by law, Plant Health Australia will not be liable for any loss, damage, cost or expense incurred in or arising by reason of any person relying on the ISSN 1838-8116 information in this publication. Readers should make and rely on their own assessment and An electronic version of this report is available for enquiries to verify the accuracy of the information provided. -

Download Full Article in PDF Format
Cryptogamie, Mycologie, 2010, 31 (4): 403-418 © 2010 Adac. Tous droits réservés Guignardia/Phyllosticta species on banana Nilam F. WULANDARI1,2, Chaiwat TO-ANUN1, Lei CAI 3, Kamel A. ABD-ELSALAM4,5,6 & Kevin D. HYDE 4,7 1Department of Plant Pathology Faculty of Agriculture, Chiang Mai University, Chiang Mai, 51200, Thailand 2Microbiology Division, Research Centre for Biology, Indonesian Institute of Sciences, Cibinong Science Centre Jl. Raya Jakarta, Bogor KM 46, Cibinong 16911, Indonesia [email protected] 3Key Laboratory of Systematic Mycology & Lichenology, Institute of Microbiology, Chinese Academy of Sciences, No. 10, North 4th Ring Road West Beijing 100190, P.R.China No. 14, Xinxi Road, Shangdi, HaiDian, Beijing, 100085, PR China 4Botany and Microbiology Department, College of Science, King Saud University, Riyadh, Saudi Arabia 5Abdul Rahman Al-Jeraisy, DNA Research Chair, College of Science, King Saud University, Riyadh, Saudi Arabia 6Plant Pathology Research Institute, Agricultural Research Centre, 9-Gamma St., Giza, Egypt 7School of Sciences, Mae Fah Luang University, 333 M. 1. T. Tasud Muang District, Chiang Rai 57100, Thailand Abstract – Guignardia musae is the reported causal agent of freckle disease of banana. The epithet has, however, been introduced on three separate occasions and only one name is valid. We therefore investigated this problem. We examined the types of G. musae Racib., G. musae F. Stevens and G. musae Syd. & P. Syd. and also made fresh collections from banana in northern Thailand. Guignardia musae Racib. is the earliest name and takes precedence over the other two names which are hononyms. G. musae F. Stevens is a different species and therefore a new name G.