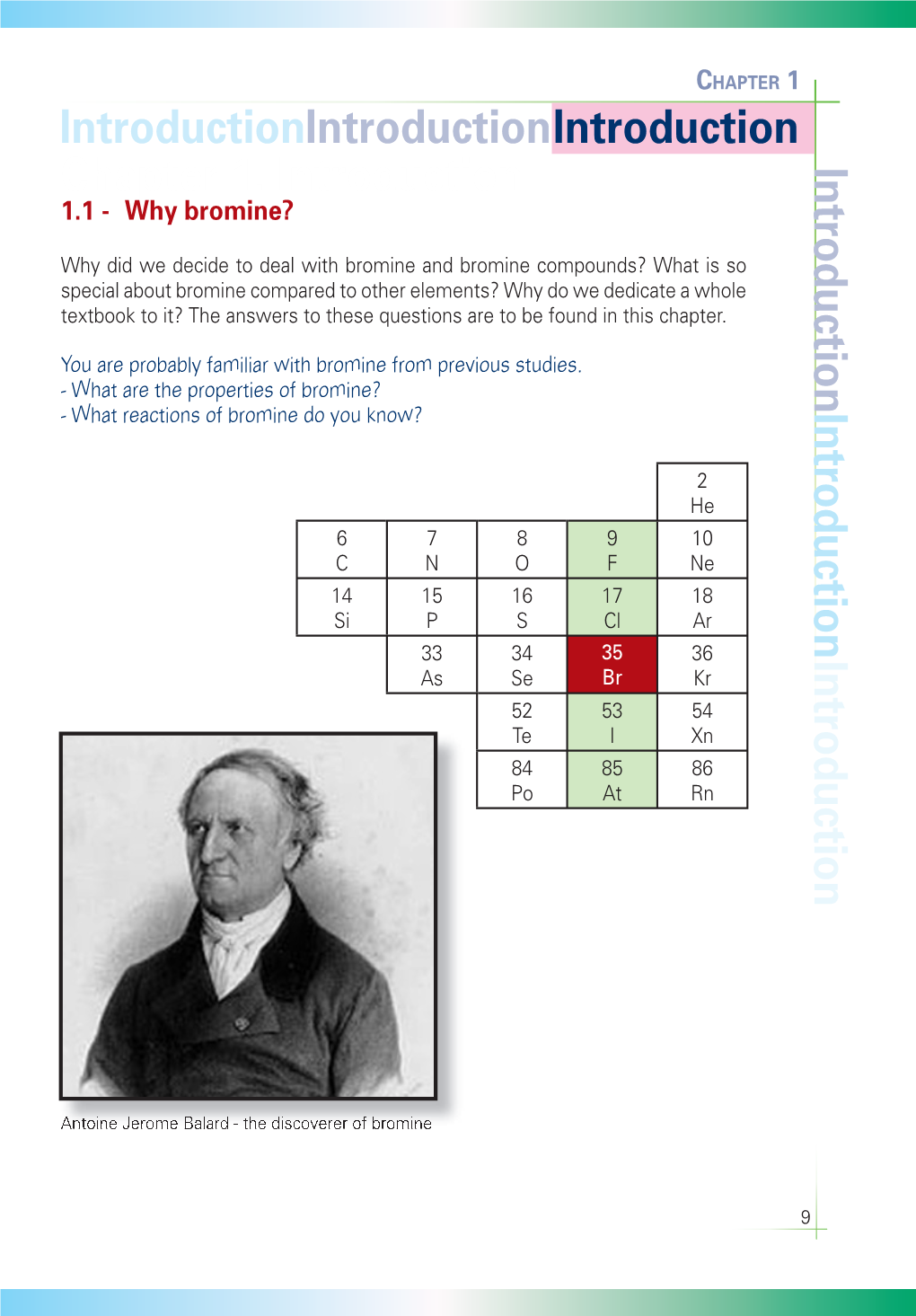
Chapter 1. Introduction Introduction 1.1 - Why Bromine?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Calcium Bromide Liquid: Safety and Handling Guide Geobrom® HG520
Calcium Bromide Liquid Safety and Handling Guide GeoBrom® HG520 Great Lakes Solutions: brominated derivative products for mercury control Contents Emergency Response Telephone Numbers 1 Responsible Care®– Doing the Right Thing 2 Introduction 3 GeoBrom® HG520 Product Description 5 Product Description Typical Product Quality Physical and Chemical Properties Environmental, Health, Safety and Regulatory Summary Regulatory Information and Training 9 Procedures for the Safe Handling of Calcium Bromide Liquid 11 Product Data Sheet Personal Protective Equipment (PPE) Safety and Handling Safety Considerations Environmental Considerations Spills Recommended Storage and Security Emergency Procedures 13 Emergency Equipment and Supplies Transportation Emergencies Fire First Aid 14 Equipment and Materials of Construction 16 Corrosion Studies Summary Shipping Containers and Transportation 17 Contact Information 19 Emergency Response Telephone Numbers for Bromine Country/ Emergency Response Provider and Region Emergency Number* CHEMTREC® United States +1-800-424-9300 or 1-703-527-3887 Carechem 24 Europe +44 (0) 1235 239 670 Carechem 24 India +91 1166 4114 05 National Registration Centre for Chemicals (NRCC) China 0532-8388-9090 *Refer to the Safety Data Sheet (SDS) for additional regional emergency response phone numbers. 1 | GLS GeoBrom® HG520 CaBr2 Liquid Safety and Handling Guide Responsible Care®– Doing the Right Thing Responsible Care® is the chemical Our Responsible Care® Policy industry’s health, safety, security, Chemtura considers ensuring the safety and health of the public, our employees, and environmental performance contractors, and customers and the protection of the environment now and improvement initiative. As an for future generations to be our core values. We are committed to meeting obligation of membership, all applicable laws, regulations, permits, and internal standards throughout Responsible Care® is the ethical the world. -

Zinc-Calcium Bromide Solution
TETRA Technologies, Inc. Material Safety Data Sheet Zinc-Calcium Bromide Solution 1. PRODUCT AND COMPANY IDENTIFICATION U.S. Toll Free (800) 424-9300 CHEMTREC (24-Hr Emergency Response): TETRA Technologies, Inc. International +1 703-527-3887 25025 I-45 North Poison Control: (800) 222-1222 The Woodlands, TX 77380 Non-emergency Customer Service: (281) 367-1983 Synonyms: Zinc-Calcium Bromide, Liquid Zinc-Calcium Bromide Chemical Family: Inorganic Salt Solution MSDS Creation Date: 08 Oct 96 MSDS Revision Date: 09 Apr 08 2. HAZARDS IDENTIFICATION NFPA Ratings: (Scale 0-4) Health: 2 Fire: 0 Reactivity: 0 WARNING: Irritant; Marine Pollutant; Explosion potential with mixtures containing potassium or sodium. Odorless, clear, colorless to amber liquid. May cause irritation to skin, eyes and respiratory system. Avoid breathing mists, EMERGENCY OVERVIEW: contact with eyes, skin and clothing. Harmful if swallowed. Do not ingest. Keep container tightly closed. Wash thoroughly after handling. Keep container tightly closed. Wash thoroughly after handling. Use only with adequate ventilation. Potential Health Effects Dermal sensitizer. Single, short exposure not likely to cause significant skin irritation. However, direct contact with solution may cause irritation, redness and Skin Contact: pain, possibly severe. Additional effects may include blistering, erythema, exfoliation, ulceration, necrosis, and scarring. May cause irritation of eyes with redness and pain and superficial injury. Additional Eye Contact: effects may include tearing, eye discharge, conjunctivitis, and blurred vision. Inhalation may cause irritation to mucous membranes and respiratory system. Inhalation: Additional effects may include coughing, labored breathing, and shortness of breath. Ingestion may cause burning sensation in mouth and throat, yellowing of the skin and eyes, nausea, vomiting, diarrhea, blood in urine, inability to urinate, low blood Ingestion: pressure, blood disorders, kidney damage, liver damage, convulsions and unconsciousness. -

Chemical Compatibility Chart for Plazit-Polygal PLAZGAL SAN Sheets
Chemical Compatibility Chart for Plazit-Polygal PLAZGAL SAN Sheets This table gives an indication of the chemical resistance of PLAZGAL SAN sheets to a range of common chemicals at 20ºC. PLAZGAL SAN sheets can be safely used with most chemicals and components such as alkalis and diluted acids, as well as with aqueous salt solutions. However, SAN has limited resistance to alcohols, aliphatic hydrocarbons, oils and fats, and is not resistant to concentrated mineral acids, aromatic and/ or halogenated hydrocarbons, esters, ethers, ketones. Important note: the chemical stability of PLAZGAL SAN sheets depends on many factors such as the concentration of the chemical agent, internal stresses in the sheets and exposure temperature, in certain situations the sheets can develop stress cracks when even when exposed to chemicals to which SAN is usually resistant. The resistance of PLAZGAL SAN sheets is indicated in the table below. Chemical Concentration Compliance Chemical Concentration Compliance Aqua Regia Dissolved Brake fluids (ATE) Affected Acetic acid To 50% Not affected Butter Not affected Acetic acid 100% Affected Butyl acetate Affected Acetone Affected Butyric acid Affected Acetylsalicylic (soln.) Not affected Cadmium (soln.) Not affected acid bromide Allyl alcohol Affected Caffeine (soln.) Not affected Almond, bitter, Not affected Calcium bromide (soln.) Not affected oil of Calcium chloride (soln.) Not affected Aluminium (soln.) Not affected Calcium (solid) Not affected chloride hypochlorite Aluminium (soln.) Not affected Calcium (soln.) -

AMC Salt Tables
AMC Salt Tables amcmud.com Table of Contents Maximum Densities 2 Potassium Acetate, KC2H3O2 9 Sodium Chloride, NaCl 3 Potassium Formate, KCOOH 10 Potassium Chloride, KC 4 Calcium Bromide, CaBr2 12 Calcium Chloride, CaCl2 5 Sodium Bromide, NaBr 13 Magnesium Chloride, MgCl2 6 Ammonium Chloride, NH4Cl 14 Potassium Sulfate, K2SO4 7 Sodium Nitrate, NaNO3 15 Sodium Formate, NaCOOH 8 Cesium Formate, CsCOOH 16 Maximum Densities 22 1836 2 1670 18 1503 SG 16 1336 lb / gal 14 1169 12 1002 1 835 4 2 3 2 2 2 Cl O 4 3 SO 2 H 2 CsCOOH ride, NH Sodium Chloride, NaCl Sodium Bromide, NaBr Sodium Bromide, Sodium Nitrate, NaNO Calcium Chloride, CaCl Calcium Bromide, CaBr Calcium Bromide, Potassium Chloride, KCl Chloride, KCl Potassium Potassium Sulfate, K Potassium Cesium Formate, Cesium Formate, Sodium Formate, NaCOOH Sodium Formate, Ammonium Chlo Magnesium Chloride, MgCl Potassium Formate, KCOOH Formate, Potassium Potassium Acetate, KC Potassium MAX DENSITY, SG MAX DENSITY, LB / GAL Ammonium Chloride, NH4Cl 1.067 8.90 Potassium Sulfate, K2SO4 1.083 9.03 Potassium Chloride, KCl 1.162 9.69 Sodium Chloride, NaCl 1.197 9.98 Magnesium Chloride, MgCl2 1.276 10.64 Sodium Nitrate, NaNO3 1.320 11.01 Sodium Formate, NaCOOH 1.330 11.06 Potassium Acetate, KC2H3O2 1.393 11.60 Calcium Chloride, CaCl2 1.396 11.64 Sodium Bromide, NaBr 1.530 12.70 Potassium Formate, KCOOH 1.580 13.18 Calcium Bromide, CaBr2 1.830 15.25 Cesium Formate, CsCOOH 2.200 18.36 amcmud.com 2 Sodium Chloride, NaCl WEIGHT DENSITY DENSITY Cl– Na+ NaCl WATER CRYST. -

(CDR) by CASRN Or Accession Number
List of Chemicals Reported for the 2012 Chemical Data Reporting (CDR) by CASRN or Accession Number For the 2012 CDR, 7,674 unique chemicals were reported by manufacturers (including importers). Chemicals are listed by CAS Registry Number (for non-confidential chemicals) or by TSCA Accession Number (for chemicals listed on the confidential portion of the TSCA Inventory). CASRN or CASRN or ACCESSION ACCESSION NUMBER CA INDEX NAME or GENERIC NAME NUMBER CA INDEX NAME or GENERIC NAME 100016 Benzenamine, 4-nitro- 10042769 Nitric acid, strontium salt (2:1) 10006287 Silicic acid (H2SiO3), potassium salt (1:2) 10043013 Sulfuric acid, aluminum salt (3:2) 1000824 Urea, N-(hydroxymethyl)- 10043115 Boron nitride (BN) 100107 Benzaldehyde, 4-(dimethylamino)- 10043353 Boric acid (H3BO3) 1001354728 4-Octanol, 3-amino- 10043524 Calcium chloride (CaCl2) 100174 Benzene, 1-methoxy-4-nitro- 100436 Pyridine, 4-ethenyl- 10017568 Ethanol, 2,2',2''-nitrilotris-, phosphate (1:?) 10043842 Phosphinic acid, manganese(2+) salt (2:1) 2,7-Anthracenedisulfonic acid, 9,10-dihydro- 100447 Benzene, (chloromethyl)- 10017591 9,10-dioxo-, sodium salt (1:?) 10045951 Nitric acid, neodymium(3+) salt (3:1) 100185 Benzene, 1,4-bis(1-methylethyl)- 100469 Benzenemethanamine 100209 1,4-Benzenedicarbonyl dichloride 100470 Benzonitrile 100210 1,4-Benzenedicarboxylic acid 100481 4-Pyridinecarbonitrile 10022318 Nitric acid, barium salt (2:1) 10048983 Phosphoric acid, barium salt (1:1) 9-Octadecenoic acid (9Z)-, 2-methylpropyl 10049044 Chlorine oxide (ClO2) 10024472 ester Phosphoric acid, -

Study of Potential Impact of Hydraulic Fracturing on Drinking Water
EPA 601/R-12/011 | December 2012 | www.epa.gov/hfstudy Study of the Potential Impacts of Hydraulic Fracturing on Drinking Water Resources PROGRESS REPORT United States Environmental Protection Agency Office of Research and Development this page intentially left blank Study of the Potential Impacts of Hydraulic Fracturing on Drinking Water Resources PROGRESS REPORT US Environmental Protection Agency Office of Research and Development Washington, DC December 2012 EPA/601/R-12/011 Study of the Potential Impacts of Hydraulic Fracturing on Drinking Water Resources: Progress Report December 2012 Disclaimer Mention of trade names or commercial products does not constitute endorsement or recommendation for use. ii Study of the Potential Impacts of Hydraulic Fracturing on Drinking Water Resources: Progress Report December 2012 Table of Contents Executive Summary .................................................................................................................................... 1 1. Introduction ......................................................................................................................................... 5 1.1. Stakeholder Engagement ...................................................................................................... 6 2. Overview of the Research Program .................................................................................................. 8 2.1. Research Questions ........................................................................................................... -

Potential Drinking Water Effects of Bromide Discharges from Coal-Fired Electric Power Plants
Potential Drinking Water Effects of Bromide Discharges from Coal-Fired Electric Power Plants Jeanne M VanBriesen, Ph.D., P.E. 1.0 Introduction Production of wastewater containing bromide is associated with fossil fuel extraction and utilization (i.e., oil and gas production and coal-fired steam electric power plants). When discharged to receiving water, these wastewaters can increase bromide concentrations in surface waters that are used as potable drinking water supplies. The presence of bromide in source waters significantly increases the formation of carcinogenic disinfection by-products (DBPs) in finished drinking water, increasing the population’s exposure to these by-products. Bromide concentrations in surface waters in the United States have typically been quite low, with average values inland ranging from 0.014-0.2 mg/L. Furthermore, bromide has a relatively high human toxicity threshold, and ecotoxicity is observed only at high concentrations. Thus, the discharge of bromide has been left largely unregulated in the United States. While naturally occurring bromide poses low risk to humans, anthropogenic brominated organic compounds are well known to exhibit significant ecotoxicity and to be associated with negative endocrine, reproductive, and carcinogenic outcomes in humans upon consumption or inhalation. Many brominated organics are used in industrial and consumer products in ways designed to limit human exposure (e.g., brominated flame retardants are applied to foam inside furniture). However, incidental production of brominated organics can occur in treating drinking water, and these compounds persist in the finished water that is consumed. Brominated disinfection by- products form when source waters containing bromide are disinfected with chemical oxidants (e.g., chlorine, ozone). -

Year 12 Chemistry Work Pack Phase IV 0.Pdf
Year 12 Chemistry Ark Globe Academy Remote Learning Pack Phase IV Monday 8 June- Friday 19 June 1 Year 12 Chemistry Session Title Work to be completed Resource Outcome On-Line provided Support 1 Recap of AS Read through content to Work pack IV Complete: Session 1 Recap of Energetics recap AS Energetics and Page(s) 3 – 8 Task 1 AS Energetics (VLE) apply to related recap questions in task 1 2 Definitions Review the table of key Work pack IV Complete: definitions and use these Page(s) 9 – 10 Task 2 to complete task 2. Textbook Page(s): 258 – 260 3 MS Teams Live lesson: Work pack IV Complete: Lesson Enthalpy of Solution Pages(s) 11 – 13 1 - DO NOW Wednesday Calculations Textbook page(s): 2 - Checking for 267 – 268 Understanding Question(s) 3 - Application Question(s) 4 Enthalpy of Complete task 3 Work pack IV Complete: Session 4 Enthalpy Solution questions using Pages(s) 14 Task 3 of Solution Calculations (VLE) Calculations information from Textbook page(s): previous session 267 – 268 5 MS Teams Live lesson: Work pack IV Complete: Lesson Born Haber Cycle Pages(s) 15 – 17 1 - DO NOW Friday Textbook page(s): 2 - Checking for 261 – 266 Understanding Question(s) 3 - Application Question(s) 6 Born Haber Complete task 4 Work pack IV Complete: Session 6 Born- Cycle questions using Pages(s) 18 Task 4 Haber Cycle Calculations (VLE) Calculations information from Textbook page(s): previous session 261 – 266 7 Application Complete a series of PPQs Work pack IV Complete: of New on the theory you have Pages(s) 19 – 22 Past paper exam questions, Theory covered since session 2 Q. -

BROMINE by Phyllis A
BROMINE By Phyllis A. Lyday International distribution of bromine extracted from the brine. As a result, there are levels until the fumigant is banned in 2001. production in 1994 was as follows: The United many bromine recovery processes built into HBFC production is frozen at 1991 levels until States, 47%; Israel, 33%; the United Kingdom, each operation. EPA proposed in the May 11, phaseout at the beginning of 1996. Great 7%; and other countries, 13%. The 1994, Federal Register to list one stream as Lakes Chemical Corp. is limited to 19,945,788 U.S. portion of world production has decreased hazardous wastes under Subtitle C of RCRA. kg of production and 15,414,746 kg for steadily since 1973, when the United States EPA added 286 chemicals to the Toxic consumption of methyl bromide. Albemarle produced 71% of the world supply. The Release Inventory (TRI) to bring the total added Corp.'s yearly baseline for production is decrease in world share has been a result of to 654 chemicals. Most of the 286 chemicals 8,233,894 kg and consumption was 6,379,906 environmental constraints and the emergence of added to the TRI list are pesticides, such as kg for methyl bromide. AmeriBrom Inc. Israel as the world's second largest producer. alachlor, aldicarb, benomyl, bromine, baseline production is 3,524,393 kg and Domestic capacity decreased 14% during the carboduran, diazinon, malathion and simazine. consumption is 4,535 kg for methyl bromide. same period. The quantity of bromine sold or EPA said that it may expand the scope of Great Lakes is limited to 45,211 kg for used in the United States was 195 million facilities that must report to include dealers and production and 40,110 kg for consumption.4 kilograms (kg) valued at $155 million. -

HDPE Chemical Resistance Guide
HDPE Chemical Resistance Guide 70º F 140º F 70º F 140º F Reagent (21º C) (60º C) Reagent (21º C) (60º C) A B Acetaldehyde S O Barium carbonate saturated S S Acetic acid (1-10%) S S Barium carbonate saturated S S Acetic acid (10-60%) S O Barium hydroxide S S Acetic acid (80-100%) S O Barium sulfate saturated S S Acetic anhydride S S Barium sulfite saturated S S Acetone S S Beer S S Acids (aromatic) S S Benzaldehyde S O Acrylic emulsions S S Benzene O U Adipic acid S S Benzene sulfonic acid S S Aluminum chloride concentrated S S Benzoic acid crystals S S Aluminum chloride dilute S S Benzoic acid saturated S S Aluminum fluoride concentrated S S Bismuth carbonate saturated S S Aluminum sulfate concentrated S S Black liquor S S Alums (all types) concentrated S S Bleach lye (10%) S S Amino acetic acid S S Borax cold saturated S S Ammonia (100% dry gas) S S Boric acid concentrated S S Ammonium acetate S S Boric acid dilute S S Ammonium bromide S S Brine S S Ammonium carbonate S S Bromic acid (10%) S S Ammonium chloride saturated S S Bromine liquid (100%) O U Ammonium fluoride (20%) S S Bromochloromethane U U Ammonium hydroxide S S Butadiene U U Ammonium metaphosphate (sat.) S S Butanediol (10%) S S Ammonium nitrate saturated S S Butanediol (60%) S S Ammonium persulfate saturated S S Butanediol (100%) S S Ammonium phosphate S S Butter S S Ammonium sulfate saturated S S Butyl acetate (100%) O U Ammonium sulfide saturated S S Butyl alcohol (100%) S S Ammonium thiocyanate saturated S S Butylene glycol S S Amyl acetate (100%) O U Butyric acid (100%) -
Calcium Bromide
Calcium bromide sc-257209 Material Safety Data Sheet Hazard Alert Code EXTREME HIGH MODERATE LOW Key: Section 1 - CHEMICAL PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME Calcium bromide STATEMENT OF HAZARDOUS NATURE CONSIDERED A HAZARDOUS SUBSTANCE ACCORDING TO OSHA 29 CFR 1910.1200. NFPA FLAMMABILITY0 HEALTH2 HAZARD INSTABILITY0 SUPPLIER Santa Cruz Biotechnology, Inc. 2145 Delaware Avenue Santa Cruz, California 95060 800.457.3801 or 831.457.3800 EMERGENCY ChemWatch Within the US & Canada: 877-715-9305 Outside the US & Canada: +800 2436 2255 (1-800-CHEMCALL) or call +613 9573 3112 SYNONYMS CaBr2, Br2Ca, "calcium dibromide" Section 2 - HAZARDS IDENTIFICATION CHEMWATCH HAZARD RATINGS Min Max Flammability 0 Toxicity 2 Body Contact 2 Min/Nil=0 Low=1 Reactivity 0 Moderate=2 High=3 Chronic 3 Extreme=4 CANADIAN WHMIS SYMBOLS EMERGENCY OVERVIEW RISK Irritating to eyes, respiratory system and skin. Harmful to aquatic organisms. POTENTIAL HEALTH EFFECTS ACUTE HEALTH EFFECTS SWALLOWED ■ Accidental ingestion of the material may be damaging to the health of the individual. ■ Bromide poisoning causes intense vomiting so the dose is often removed. Effects include drowsiness, irritability, inco-ordination, vertigo, confusion, mania, hallucinations and coma. EYE ■ This material can cause eye irritation and damage in some persons. SKIN ■ This material can cause inflammation of the skin oncontact in some persons. ■ The material may accentuate any pre-existing dermatitis condition. ■ Skin contact is not thought to have harmful health effects (as classified under EC Directives); the material may still produce health damage following entry through wounds, lesions or abrasions. ■ Open cuts, abraded or irritated skin should not be exposed to this material. -

An Efficient Synthesis of Β-Enaminoketone/Ester Under Grinding Condition Using Calcium Bromide As Solid Support Catalyst
Available online at www.derpharmachemica.com ISSN 0975-413XCODEN Der Pharma Chemica, 2017, 9(8):55-58 (USA): PCHHAX (http://www.derpharmachemica.com/archive.html) An Efficient Synthesis of β-Enaminoketone/Ester Under Grinding Condition Using Calcium Bromide as Solid Support Catalyst Pramod Kulkarni*, Ajay Jadhav Department of Chemistry, Hutatma Rajguru Mahavidyalaya, Rajgurunagar, Pune, Maharashtra, 410505, India ABSTRACT Calcium bromide was used as a catalyst in the synthesis of β-enaminoketone/ester from 1,3-dicarbonyl compound and amines. The reaction was carried out in mortar and pestle at room temperature. The process is mild, efficient, eco-friendly and cost-effective with the use of very small amount of catalyst; avoiding use solvent, simple product separation, and high yield. We compare this grinding reaction condition with reaction in solvent and we got better results with the grinding reaction condition. Keywords: Calcium bromide, β-Enaminoketone, Solvent free, Grindstone Chemistry, Green synthesis INTRODUCTION Solid-state chemistry has been developed rapidly as a competent and selective method, since molecules in the crystals have been arranged tightly and regularly. One of the methods belonging to this etiquette is a simple grinding method called a grindstone (mechanochemical) method which reactants are ground simply by mortar and pestle. Grinding is attracting many organic chemists due to it is performed in the absence of solvent, leading to safe and environmentally friendly synthesis. In addition to this, the proposed technique does not require external heating, leading to energy efficient synthesis and may be regarded as more cost-effective and ecologically favourable procedure in chemistry. Mechanical-induced breaking of molecular bonds, reduction of particle size, increase in surface area, formation of defects and local melting occur due to the kinetic energy supplied during grinding in solvent-free organic reactions based on grinding.