Compositional and Mutational Rate Heterogeneity in Mitochondrial Genomes and Its Effect on the Phylogenetic Inferences of Cimico
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Proceedings of the United States National Museum
Proceedings of the United States National Museum SMITHSONIAN INSTITUTION • WASHINGTON, D.C. Volume 112 I960 Number 3431 LACE-BUG GENERA OF THE WORLD (HEMIPTERA: TINGIDAE) « By Carl J. Drake and Florence A. Ruhoff Introduction A treatise of the generic names of the family Tingidae from a global standpoint embodies problems similar to those frequently encountered in corresponding studies in other animal groups. The more im- portant criteria, including such basic desiderata as fixation of type species, synonyms, priority, and dates of technical publications implicate questions concomitant with recent trends toward the clarification and stabilization of zoological nomenclature. Zoogeography, predicated and authenticated on the generic level by the distribution of genera and species, is portrayed here by means of tables, charts, and maps of the tingifauna of the world. This visual pattern of distribution helps one to form a more vivid concept of the family and its hierarchic levels of subfamilies and genera. To a limited extent the data indicate distributional concentrations and probable centers of evolution and dispersal paths of genera. The phylogenetic relationship of genera is not discussed. The present treatise recognizes 216 genera (plus 79 synonyms, homonyms, and emendations) of the Tingidae of the world and gives 1 Research for this paper was supported In part by the National Science Foundation, grant No. 4095. 2 PROCEEDINGS OF THE NATIONAL MUSEUM vol. 112 the figure of 1,767 as the approximate number of species now recog- nized. These figures, collated with similar categories in Lethierry and Severin (1896), show that there has been an increase of many genera and hundreds of species of Tingidae during the past three- quarters of a century. -

The Complete Mitochondrial Genome of the Damsel Bug Alloeorhynchus
Int. J. Biol. Sci. 2012, 8 93 Ivyspring International Publisher International Journal of Biological Sciences 2012; 8(1):93-107 Research Paper The Complete Mitochondrial Genome of the Damsel Bug Alloeorhynchus bakeri (Hemiptera: Nabidae) Hu Li1,*, Haiyu Liu1,*, Liangming Cao2, Aimin Shi1, Hailin Yang1, Wanzhi Cai1 1. Department of Entomology, China Agricultural University, Beijing 100193, China 2. The Key Laboratory of Forest Protection of China State Forestry Administration, Research Institute of Forest Ecology, Environment and Protection, Chinese Academy of Forestry, Beijing 100091, China * These authors contributed equally to this work. Corresponding author: Dr. Wanzhi Cai, Department of Entomology, China Agricultural University, Yuanmingyuan West Road, Beijing 100193, China. Phone: 86-10-62732885; Fax: 86-10-62732885; Email: [email protected] © Ivyspring International Publisher. This is an open-access article distributed under the terms of the Creative Commons License (http://creativecommons.org/ licenses/by-nc-nd/3.0/). Reproduction is permitted for personal, noncommercial use, provided that the article is in whole, unmodified, and properly cited. Received: 2011.09.17; Accepted: 2011.11.10; Published: 2011.11.24 Abstract The complete sequence of the mitochondrial DNA (mtDNA) of the damsel bug, Al- loeorhynchus bakeri, has been completed and annotated in this study. It represents the first sequenced mitochondrial genome of heteropteran family Nabidae. The circular genome is 15, 851 bp in length with an A+T content of 73.5%, contains the typical 37 genes that are arranged in the same order as that of the putative ancestor of hexapods. Nucleotide composition and codon usage are similar to other known heteropteran mitochondrial genomes. -

Hemiptera: first Record for an Australian Lophopid (Hemiptera, Lophopidae)
Australian Journal of Entomology (2007) 46, 129–132 Historical use of substrate-borne acoustic production within the Hemiptera: first record for an Australian Lophopid (Hemiptera, Lophopidae) Adeline Soulier-Perkins,1* Jérôme Sueur2 and Hannelore Hoch3 1Muséum National d’Histoire Naturelle, Département Systématique et Évolution, USM 601 MNHN & UMR 5202 CNRS, Case Postale 50, 45, Rue Buffon, F-75005 Paris, France. 2NAMC-CNRS UMR 8620, Bât. 446, Université Paris XI, F-91405 Orsay Cedex, France. 3Museum für Naturkunde, Institut für Systematische Zoologie, Humboldt-Universität zu Berlin Invalidenstr. 43, D- 10115 Berlin, Germany. Abstract Here the first record of communication through substrate-borne vibrations for the Lophopidae family is reported. The signals from Magia subocellata that the authors recorded were short calls with a decreasing frequency modulation. Acoustic vibrations have been observed for other families within the Hemiptera and a scenario concerning the historical use of vibrational communication within the Hemiptera is tested using a phylogenetic inference. The most parsimonious hypothesis suggests that substrate-borne communication is ancestral for the hemipteran order and highlights the groups for which future acoustic research should be undertaken. Key words Cicadomorpha, Coleorrhyncha, evolutionary scenario, Heteroptera, Sternorrhyncha, substrate vibration. INTRODUCTION Lophopidae migrating into America via the Bering land bridge. Some other ancestors of the extant groups moved onto Many animals have been recently recognised for their ability newly emerging land in the Pacific, expanding their distribu- to communicate through substrate-borne vibrations (Hill tion as far east as the Samoan Islands, and as far south as 2001). While elephants produce vibrations transmitted by the Australia (Soulier-Perkins 2000). -

A THESIS for the DEGREE of DOCTOR of PHILOSOPHY By
A THESIS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY Systematic review of subfamily Phylinae (Hemiptera: Miridae) in Korean Peninsula with molecular phylogeny of Miridae By Ram Keshari Duwal Program in Entomology Department of Agricultural Biotechnology Seoul National University February, 2013 Systematic review of subfamily Phylinae (Hemiptera: Miridae) in Korean Peninsula with molecular phylogeny of Miridae UNDER THE DIRECTION OF ADVISER SEUNGHWAN LEE SUBMITTED TO THE FACULTY OF THE GRADUATE SCHOOL OF SEOUL NATIONAL UNIVERSIITY By Ram Keshari Duwal Program in Entomology Department of Agricultural Biotechnology Seoul National University February, 2013 APRROVED AS A QUALIFIED DISSERTATION OF RAM KESHARI DUWAL FOR THE DEGREE OF DOCTOR OF PHILOSOPHY BY THE COMMITTEE MEMBERS CHAIRMAN Si Hyeock Lee VICE CHAIRMAN Seunghwan Lee MEMBER Young-Joon Ahn MEMBER Yang-Seop Bae MEMBER Ki-Jeong Hong ABSTRACT Systematic review of subfamily Phylinae (Hemiptera: Miridae) in Korean Peninsula with molecular phylogeny of Miridae Ram Keshari Duwal Program of Entomology, Department of Agriculture Biotechnology The Graduate School Seoul National University The study conducted two themes: (1) The systematic review of subfamily Phylinae (Heteroptera: Miridae) in Korean Peninsula, with brief zoogeographic discussion in East Asia, and (2) Molecular phylogeny of Miridae: (i) Higher group relationships within family Miridae, and (ii) Phylogeny of subfamily Phylinae. In systematic review a total of eighty four species in twenty eight genera of Phylines are recognized from the Korean Peninsula. During this study, twenty new reports including six new species were investigated; and purposed a synonym and revised recombination. Keys to genera and species, diagnosis, descriptions including male and female genitalia, illustrations and short biological notes are provided for each of the species. -

The Semiaquatic Hemiptera of Minnesota (Hemiptera: Heteroptera) Donald V
The Semiaquatic Hemiptera of Minnesota (Hemiptera: Heteroptera) Donald V. Bennett Edwin F. Cook Technical Bulletin 332-1981 Agricultural Experiment Station University of Minnesota St. Paul, Minnesota 55108 CONTENTS PAGE Introduction ...................................3 Key to Adults of Nearctic Families of Semiaquatic Hemiptera ................... 6 Family Saldidae-Shore Bugs ............... 7 Family Mesoveliidae-Water Treaders .......18 Family Hebridae-Velvet Water Bugs .......20 Family Hydrometridae-Marsh Treaders, Water Measurers ...22 Family Veliidae-Small Water striders, Rime bugs ................24 Family Gerridae-Water striders, Pond skaters, Wherry men .....29 Family Ochteridae-Velvety Shore Bugs ....35 Family Gelastocoridae-Toad Bugs ..........36 Literature Cited ..............................37 Figures ......................................44 Maps .........................................55 Index to Scientific Names ....................59 Acknowledgement Sincere appreciation is expressed to the following individuals: R. T. Schuh, for being extremely helpful in reviewing the section on Saldidae, lending specimens, and allowing use of his illustrations of Saldidae; C. L. Smith for reading the section on Veliidae, checking identifications, and advising on problems in the taxon omy ofthe Veliidae; D. M. Calabrese, for reviewing the section on the Gerridae and making helpful sugges tions; J. T. Polhemus, for advising on taxonomic prob lems and checking identifications for several families; C. W. Schaefer, for providing advice and editorial com ment; Y. A. Popov, for sending a copy ofhis book on the Nepomorpha; and M. C. Parsons, for supplying its English translation. The University of Minnesota, including the Agricultural Experi ment Station, is committed to the policy that all persons shall have equal access to its programs, facilities, and employment without regard to race, creed, color, sex, national origin, or handicap. The information given in this publication is for educational purposes only. -

Download Download
Journal Journal of Entomological of Entomological and andAcarological Acarological Research Research 2018; 2012; volume volume 50:7836 44:e A review of sulfoxaflor, a derivative of biological acting substances as a class of insecticides with a broad range of action against many insect pests L. Bacci,1 S. Convertini,2 B. Rossaro3 1Dow Agrosciences Italia, Bologna; 2ReAgri srl, Massafra (TA); 3Department of Food, Environmental and Nutritional Sciences, University of Milan, Italy Abstract Introduction Sulfoxaflor is an insecticide used against sap-feeding insects Insecticides are important tools in the control of insect pests. (Aphididae, Aleyrodidae) belonging to the family of sulfoximine; An unexpected unfavourable consequence of the increased use of sulfoximine is a chiral nitrogen-containing sulphur (VI) molecule; insecticides was the reduction of pollinator species and the subse- it is a sub-group of insecticides that act as nicotinic acetylcholine quent declines in crop yields. Multiple factors in various combina- receptor (nAChR) competitive modulators. Sulfoxaflor binds to tions as modified crops, habitat fragmentation, introduced dis- nAChR in place of acetylcholine and acts as an allosteric activator eases and parasites, including mites, fungi, virus, reduction in for- of nAChR. Thanks to its mode of action resistance phenomena are age, poor nutrition, and onlyqueen failure were other probable contrib- uncommon, even few cases of resistance were reported. It binds to utory causes of elevated colony loss of pollinator species, but the receptors determining uncontrolled nerve impulses followed by reduction of pollinator species was often attributed to some class- muscle tremors to which paralysis and death follows. Sulfoxaflor es of insecticides. acts on the same receptors of neonicotinoids as nicotine and In an effortuse to reduce the unfavourable consequences of an butenolides, but it binds differently. -

TINGIDAE of NEW GUINEA (Hemiptera)
Pacific Insects 2 (3) : 339-380 October 10, 1960 TINGIDAE OF NEW GUINEA (Hemiptera) By Carl J. Drake SMITHSONIAN INSTITUTION, WASHINGTON, D. C. This is a preliminary account of the Family Tingidae in New Guinea and nearby is lands. The new records herein are based upon the following collections : Bernice P. Bi shop Museum, specimens netted by Dr. J. Linsley Gressitt and associates in the present field survey of the insects of the islands of South Pacific Ocean1; American Museum of Natural History (New York); Rijksmuseum van Natuurlijke Historie (Leiden); and Drake Collection (USNM). Specimens also have been received from J. J. H. SzenMvany, Port Moresby, and other entomologists in New Guinea and nearby islands. The records in the literature are also included. From these various sources, a sizeable representation of the tingifauna of New Guinea has been available for initial study. The records are of particular value because they contribute immensely to the poorly-known fauna of this large island lying in a zoological realm of such unusual chorological and scientific interest. The genera and their compo nents provide biotic data relative to Systematics, affinities, and dispersal of lace-bugs need ed for comparative, faunistic studies with those of the other large islands of the Eastern Hemisphere, such as Madagascar, Taiwan, Ceylon, and Luzon. To some extent, the main lands of Australia, India, and Africa also enter into the New Guinean faunistic picture. In comprehensive study of the morphology and higher classification of tingids, Drake and Davis (1960) : (1) Erected the Superfamily Miroidea Hahn to hold the families Tin gidae Laporte and Miridae Hahn; (2) Divided the family Tingidae into the subfamilies Tinginae Laporte, Cantacaderinae Stal, and Vianaidinae Kormilev; (3) Separated the Cantacaderinae into the tribes Cantacaderini and Phatnomini; and (4) Suppressed the Sub family Agrammatinae Douglas and Scott (= Serenthiinae Stal) as a synonym of the Sub family Tinginae. -

Hemiptera, Prosorrhyncha) with Special Reference to the Pregenital Abdominal Structure1
© Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at Justification for the Aradimorpha as an infraorder of the suborder Heteroptera (Hemiptera, Prosorrhyncha) with Special Reference to the Pregenital Abdominal Structure1 M.H. SWEET Abstract: Aradomorpha SWEET 1996 is replaced with Aradimorpha because of homonymy with Arado- morpha CHAMPION 1899, a genus of Reduviidae. The Aradimorpha differ from the Pentatomomorpha s.s. and the Leptopodomorpha in having a plesiomorphic connexivum of dorsal epipleurites and ventral hy- popleurites rather than having the connexivum turned over so that the hypopleurites are dorsalized and the epipleurites folded into the abdomen. In most Aradimorpha, in both males and females, sterna 3 to 7 are free with intersegmental conjunctiva; terga 1-2 and 3 to 6 are united, but all epipleurites are free. In the Pentatomomorpha at least abdominal sterna 2 to 4 in females and sterna 2 to 5 in males are uni- ted or fused without conjunctiva. In some aradids the hypopleurites are united or fused with the sterna, but hypopleurite 2 is usually free. Sternum 2 is sometimes united to fused with sternum 1 and the meta- sternum. The abdominal spiracles in the Aradimorpha are ventral on the hypopleurites, although some- times very lateral in position on the hypopleurites, with the exception of the Chinamyersiini in which spiracles 4, 5 and 6 are dorsal on the epipleurites in Chinamyersia, and 5 and 6 dorsal in Gnostocoris, whi- le in the Tretocorini (Tretocoris and Kumaressa) spiracle 2 seems dorsal but is actually very lateral on the hypopleurite. In the Termitaphididae, epipleurites and hypopleurites are distinct, forming mobile lateral abdominal lobes. -

Scope: Munis Entomology & Zoology Publishes a Wide Variety of Papers
_____________Mun. Ent. Zool. Vol. 11, No. 2, June 2016__________ 501 REDUVIIDAE (HETEROPTERA: HEMIPTERA) RECORDED AS NEW FROM ODISHA, INDIA Paramita Mukherjee* and M. E. Hassan* * Zoological Survey of India, ‘M’ Block, New Alipore, Kolkata-700053, INDIA. E-mails: [email protected]; [email protected] [Mukherjee, P. & Hassan, M. E. 2016. Reduviidae (Heteroptera: Hemiptera) recorded as new from Odisha, India. Munis Entomology & Zoology, 11 (2): 501-507] ABSTRACT: The paper presents ten new records viz. Rhynocoris squalus (Distant), Staccia diluta (Stal), Oncocephalus notatus Klug, Oncocephalus fuscinotum Reuter, Ectrychotes dispar Reuter, Androclus pictus (Herr-Schiff), Ectomocoris tibialis Distant, Lisarda annulosa Stal, Acanthaspis quinquespinosa (Fabricius) and Acanthaspis flavipes Stal of the family Reduviidae from the state of Odisha, India. General characters of the group, keys to various taxa, diagnostic characters, synonymies, distribution in India and elsewhere under each species are also provided. KEY WORDS: Hemiptera, Reduviidae, Odisha The members of the family Reduviidae are commonly known as “Assassin bugs”. Most of the species of Reduviidae are nocturnal. The family Reduviidae belongs to the superfamily Reduvoidea of the suborder Heteroptera under the order Hemiptera of class Insecta. Their large size and aggressive nature enable them to predate and eat many insects. With more than 6878 described species and subspecies under 981 genera belonging to 25 subfamilies of the family Reduviidae recorded from the world (Henry, 2009) are one of the largest and morphologically most diverse group of Heteroptera or true bugs. Of which, 465 species under 144 genera belonging to 14 subfamilies are recorded from India (Biswas and Mitra, 2011). Distant (1904, 1910) recorded three species from Berhampur, Odisha viz Acanthaspis rama Distant of Reduviinae, Ectomocoris ochropterus Stal and Peirates flavipes (Walker) of Peiratinae. -
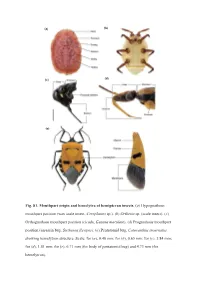
Page 1 (A) (E) Fig. S1. Mouthpart Origin and Hemelytra of Hemipteran
(a) (b) (d) (c) (e) Fig 61. Mouthpart origin and hemelytra of hemipteran insects. (a) Hypognathous mouthpart position (wax scale insect, Ceroplastes sp.). (b) Orthezia sp. (scale insect). (c) Orthognathous mouthpart position (cicada, Gaeana maculate). (d) Prognathous mouthpart position (assassin bug, Sirthenea flavipes). (e) Pentatomid bug, Catacanthus incarnatus showing hemelytron structure. Scale: for (a), 0.40 mm; for (b), 0.65 mm; for (c), 3.84 mm; for (d), 1.81 mm; for (e), 6.71 mm (for body of pentatomid bug) and 4.73 mm (for hemelytron). Sternorrhyncha Cicadomorpha Fulgoromorpha Coleorrhyncha Heteroptera PCG1 PCG2 Sternorrhyncha Cicadomorpha Fulgoromorpha Coleorrhyncha Heteroptera PCG3 RNA )LJ62. AliGROOVE analysis for codon positions of protein-coding genes (PCGs) and RNA genes. PCG1, the first codon position of PCGs. PCG2, the second codon position of PCGs. PCG3, the third codon position of PCGs. RNA, sequences of tRNA and rRNA genes. The mean similarity score between sequences is represented by a colored square, based on AliGROOVE scores from -1, indicating great difference in rates from the remainder of the data set, that is, heterogeneity (red coloring), to +1, indicating that ratesmatch all other comparisons (blue coloring). Bactericera sinica Sternorrhyncha Cicadomorpha Coleorrhyncha Fulgoromorpha Dipsocoromorpha Gerromorpha Enicocephalomorpha Nepomorpha Leptopodomorpha Cimicomorpha Heteroptera Pentatomomorpha Eusthenes cupreus FigS33K\ORJHQHWLFWUHHLQIHUUHGIURP3K\OR%D\HVDQDO\VLVRIWKH3&*51$GDWDVHWXQGHUWKe &$7*75PL[WXUHPRGHO9DOXHVDWQRGHVDUH%D\HVLDQ33V -

Insecta: Hemiptera: Heteroptera)
© Zool.-Bot. Ges. Österreich, Austria; download unter www.zobodat.at Acta ZooBot Austria 155, 2018, 251–256 Snapshot of the terrestrial true bug fauna of the Pocem floodplains (Insecta: Hemiptera: Heteroptera) Wolfgang Rabitsch 61 terrestrial true bug (Heteroptera) species are reported from a short field trip in April 2017 along the river Vjosa in the Pocem floodplains, Albania. Five species are reported for the first time for Albania, indicating insufficient baseline information on the distribution of true bugs in the region. Future sampling designs should consider the interstitial habitats, river gravel and sand banks, and adjacent dry grassland areas. Certain difficulties aside, true bugs are a significant group of insect species with de- scriptive and indicative value. RABITSCH W., 2018: Momentaufnahme der terrestrischen Wanzenfauna im Pocem Überschwemmungsgebiet (Insecta: Hemiptera: Heteroptera). Während einer kurzen Exkursion im April 2017 entlang des Flusses Vjosa im Pocem Überschwemmungsgebiet, Albanien, wurden 61 terrestrische Wanzenarten (Heterop- tera) festgestellt. Fünf Arten werden der erste Mal für Albanien gemeldet, ein Hinweis auf die unzureichende Datenlage der Verbreitung von Wanzen im Gebiet. Zukünftige Erhebungen sollten insbesondere die interstitiellen Habitate am Flussufer, Sandbänke und die angrenzenden Trockenrasenstandorte untersuchen. Trotz gewisser Schwierig- keiten sind Wanzen als Deskriptoren und Indikatoren der Lebensräume eine aussage- kräftige Insektengruppe von hohem Wert. Keywords: Albania, floodplain, Heteroptera, new records, riparian, river. Introduction True bugs (Insecta: Hemiptera: Heteroptera) are well-known descriptors and indicators of terrestrial and aquatic habitats and their ecological quality (Duelli & Obrist 1998, Achtziger et al. 2007, Rabitsch 2008, Skern et al. 2010). Due to their diversity of feeding habits, life-histories, biology, and preferred habitats, true bugs are a meaningful addition to any Environmental Impact Assessment (e.g. -

An Annotated Catalog of the Iranian Miridae (Hemiptera: Heteroptera: Cimicomorpha)
Zootaxa 3845 (1): 001–101 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Monograph ZOOTAXA Copyright © 2014 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3845.1.1 http://zoobank.org/urn:lsid:zoobank.org:pub:C77D93A3-6AB3-4887-8BBB-ADC9C584FFEC ZOOTAXA 3845 An annotated catalog of the Iranian Miridae (Hemiptera: Heteroptera: Cimicomorpha) HASSAN GHAHARI1 & FRÉDÉRIC CHÉROT2 1Department of Plant Protection, Shahre Rey Branch, Islamic Azad University, Tehran, Iran. E-mail: [email protected] 2DEMNA, DGO3, Service Public de Wallonie, Gembloux, Belgium, U. E. E-mail: [email protected] Magnolia Press Auckland, New Zealand Accepted by M. Malipatil: 15 May 2014; published: 30 Jul. 2014 HASSAN GHAHARI & FRÉDÉRIC CHÉROT An annotated catalog of the Iranian Miridae (Hemiptera: Heteroptera: Cimicomorpha) (Zootaxa 3845) 101 pp.; 30 cm. 30 Jul. 2014 ISBN 978-1-77557-463-7 (paperback) ISBN 978-1-77557-464-4 (Online edition) FIRST PUBLISHED IN 2014 BY Magnolia Press P.O. Box 41-383 Auckland 1346 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2014 Magnolia Press All rights reserved. No part of this publication may be reproduced, stored, transmitted or disseminated, in any form, or by any means, without prior written permission from the publisher, to whom all requests to reproduce copyright material should be directed in writing. This authorization does not extend to any other kind of copying, by any means, in any form, and for any purpose other than private research use. ISSN 1175-5326 (Print edition) ISSN 1175-5334 (Online edition) 2 · Zootaxa 3845 (1) © 2014 Magnolia Press GHAHARI & CHÉROT Table of contents Abstract .