Systematics of Disakisperma (Poaceae, Chloridoideae, Chlorideae)
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Ajo Peak to Tinajas Altas: a Flora of Southwestern Arizona
Felger, R.S., S. Rutman, and J. Malusa. 2014. Ajo Peak to Tinajas Altas: A flora of southwestern Arizona. Part 6. Poaceae – grass family. Phytoneuron 2014-35: 1–139. Published 17 March 2014. ISSN 2153 733X AJO PEAK TO TINAJAS ALTAS: A FLORA OF SOUTHWESTERN ARIZONA Part 6. POACEAE – GRASS FAMILY RICHARD STEPHEN FELGER Herbarium, University of Arizona Tucson, Arizona 85721 & Sky Island Alliance P.O. Box 41165, Tucson, Arizona 85717 *Author for correspondence: [email protected] SUSAN RUTMAN 90 West 10th Street Ajo, Arizona 85321 JIM MALUSA School of Natural Resources and the Environment University of Arizona Tucson, Arizona 85721 [email protected] ABSTRACT A floristic account is provided for the grass family as part of the vascular plant flora of the contiguous protected areas of Organ Pipe Cactus National Monument, Cabeza Prieta National Wildlife Refuge, and the Tinajas Altas Region in southwestern Arizona. This is the second largest family in the flora area after Asteraceae. A total of 97 taxa in 46 genera of grasses are included in this publication, which includes ones established and reproducing in the modern flora (86 taxa in 43 genera), some occurring at the margins of the flora area or no long known from the area, and ice age fossils. At least 28 taxa are known by fossils recovered from packrat middens, five of which have not been found in the modern flora: little barley ( Hordeum pusillum ), cliff muhly ( Muhlenbergia polycaulis ), Paspalum sp., mutton bluegrass ( Poa fendleriana ), and bulb panic grass ( Zuloagaea bulbosa ). Non-native grasses are represented by 27 species, or 28% of the modern grass flora. -

Guidelines for Using the Checklist
Guidelines for using the checklist Cymbopogon excavatus (Hochst.) Stapf ex Burtt Davy N 9900720 Synonyms: Andropogon excavatus Hochst. 47 Common names: Breëblaarterpentyngras A; Broad-leaved turpentine grass E; Breitblättriges Pfeffergras G; dukwa, heng’ge, kamakama (-si) J Life form: perennial Abundance: uncommon to locally common Habitat: various Distribution: southern Africa Notes: said to smell of turpentine hence common name E2 Uses: used as a thatching grass E3 Cited specimen: Giess 3152 Reference: 37; 47 Botanical Name: The grasses are arranged in alphabetical or- Rukwangali R der according to the currently accepted botanical names. This Shishambyu Sh publication updates the list in Craven (1999). Silozi L Thimbukushu T Status: The following icons indicate the present known status of the grass in Namibia: Life form: This indicates if the plant is generally an annual or G Endemic—occurs only within the political boundaries of perennial and in certain cases whether the plant occurs in water Namibia. as a hydrophyte. = Near endemic—occurs in Namibia and immediate sur- rounding areas in neighbouring countries. Abundance: The frequency of occurrence according to her- N Endemic to southern Africa—occurs more widely within barium holdings of specimens at WIND and PRE is indicated political boundaries of southern Africa. here. 7 Naturalised—not indigenous, but growing naturally. < Cultivated. Habitat: The general environment in which the grasses are % Escapee—a grass that is not indigenous to Namibia and found, is indicated here according to Namibian records. This grows naturally under favourable conditions, but there are should be considered preliminary information because much usually only a few isolated individuals. -

Texas Forts Trail Region
CatchCatch thethe PioPionneereer SpiritSpirit estern military posts composed of wood and While millions of buffalo still roamed the Great stone structures were grouped around an Plains in the 1870s, underpinning the Plains Indian open parade ground. Buildings typically way of life, the systematic slaughter of the animals had included separate officer and enlisted troop decimated the vast southern herd in Texas by the time housing, a hospital and morgue, a bakery and the first railroads arrived in the 1880s. Buffalo bones sutler’s store (provisions), horse stables and still littered the area and railroads proved a boon to storehouses. Troops used these remote outposts to the bone trade with eastern markets for use in the launch, and recuperate from, periodic patrols across production of buttons, meal and calcium phosphate. the immense Southern Plains. The Army had other motivations. It encouraged Settlements often sprang up near forts for safety the kill-off as a way to drive Plains Indians onto and Army contract work. Many were dangerous places reservations. Comanches, Kiowas and Kiowa Apaches with desperate characters. responded with raids on settlements, wagon trains and troop movements, sometimes kidnapping individuals and stealing horses and supplies. Soldiers stationed at frontier forts launched a relentless military campaign, the Red River War of 1874–75, which eventually forced Experience the region’s dramatic the state’s last free Native Americans onto reservations in present-day Oklahoma. past through historic sites, museums and courthouses — as well as historic downtowns offering unique shopping, dining and entertainment. ★★ ★★ ★★ ★★ ★★ ★★ ★★ 2 The westward push of settlements also relocated During World War II, the vast land proved perfect cattle drives bound for railheads in Kansas and beyond. -

The Civilian Conservation Corps and the National Park Service, 1933-1942: an Administrative History. INSTITUTION National Park Service (Dept
DOCUMENT RESUME ED 266 012 SE 046 389 AUTHOR Paige, John C. TITLE The Civilian Conservation Corps and the National Park Service, 1933-1942: An Administrative History. INSTITUTION National Park Service (Dept. of Interior), Washington, D.C. REPORT NO NPS-D-189 PUB DATE 85 NOTE 293p.; Photographs may not reproduce well. PUB TYPE Reports - Descriptive (141) -- Historical Materials (060) EDRS PRICE MF01/PC12 Plus Postage. DESCRIPTORS *Conservation (Environment); Employment Programs; *Environmental Education; *Federal Programs; Forestry; Natural Resources; Parks; *Physical Environment; *Resident Camp Programs; Soil Conservation IDENTIFIERS *Civilian Conservation Corps; Environmental Management; *National Park Service ABSTRACT The Civilian Conservation Corps (CCC) has been credited as one of Franklin D. Roosevelt's most successful effortsto conserve both the natural and human resources of the nation. This publication provides a review of the program and its impacton resource conservation, environmental management, and education. Chapters give accounts of: (1) the history of the CCC (tracing its origins, establishment, and termination); (2) the National Park Service role (explaining national and state parkprograms and co-operative planning elements); (3) National Park Servicecamps (describing programs and personnel training and education); (4) contributions of the CCC (identifying the major benefits ofthe program in the areas of resource conservation, park and recreational development, and natural and archaeological history finds); and (5) overall -

RV Sites in the United States Location Map 110-Mile Park Map 35 Mile
RV sites in the United States This GPS POI file is available here: https://poidirectory.com/poifiles/united_states/accommodation/RV_MH-US.html Location Map 110-Mile Park Map 35 Mile Camp Map 370 Lakeside Park Map 5 Star RV Map 566 Piney Creek Horse Camp Map 7 Oaks RV Park Map 8th and Bridge RV Map A AAA RV Map A and A Mesa Verde RV Map A H Hogue Map A H Stephens Historic Park Map A J Jolly County Park Map A Mountain Top RV Map A-Bar-A RV/CG Map A. W. Jack Morgan County Par Map A.W. Marion State Park Map Abbeville RV Park Map Abbott Map Abbott Creek (Abbott Butte) Map Abilene State Park Map Abita Springs RV Resort (Oce Map Abram Rutt City Park Map Acadia National Parks Map Acadiana Park Map Ace RV Park Map Ackerman Map Ackley Creek Co Park Map Ackley Lake State Park Map Acorn East Map Acorn Valley Map Acorn West Map Ada Lake Map Adam County Fairgrounds Map Adams City CG Map Adams County Regional Park Map Adams Fork Map Page 1 Location Map Adams Grove Map Adelaide Map Adirondack Gateway Campgroun Map Admiralty RV and Resort Map Adolph Thomae Jr. County Par Map Adrian City CG Map Aerie Crag Map Aeroplane Mesa Map Afton Canyon Map Afton Landing Map Agate Beach Map Agnew Meadows Map Agricenter RV Park Map Agua Caliente County Park Map Agua Piedra Map Aguirre Spring Map Ahart Map Ahtanum State Forest Map Aiken State Park Map Aikens Creek West Map Ainsworth State Park Map Airplane Flat Map Airport Flat Map Airport Lake Park Map Airport Park Map Aitkin Co Campground Map Ajax Country Livin' I-49 RV Map Ajo Arena Map Ajo Community Golf Course Map -

GOOSE ISLAND STATE PARK STATE ISLAND GOOSE Concession Building
PARKS TO VIEW CCC WORK Born out of the Great Depression, the Civilian Conservation Corps put young men to work in the 1930s. The jobs involved building parks and conserving natural resources across the country. Many of our state parks here in Texas display the CCC’s handiwork. Texas now has 29 CCC state parks. Some, like Garner and Palo Duro Canyon, are well known to travelers across the state. So here’s a list of some CCC parks you may not have visited … yet. By Dale Blasingame � PHOTO BY EARL NOTTINGHAM / TPWD PHOTO © LAURENCEPHOTO PARENT ABILENE STATE PARK PARKS TO VIEW CCC WORK Map and directions CCC enrollees used native limestone and 150 Park Road 32 red sandstone to build many of the park’s Tuscola, TX 79562 features, including the arched concession building (with observation tower) and the Latitude: 32.240731 water tower. The CCC also constructed the Longitude: -99.879139 swimming pool, with pyramidal poolside pergolas. Online reservations (325) 572-3204 Entrance Fees Adult Day Use: $5 Daily Child 12 and Under: Free Visit park website PHOTO BY TPWD BY PHOTO BONHAM STATE PARK PARKS TO VIEW CCC WORK Map and directions The CCC touch can be seen everywhere – 1363 State Park 24 from the earthen dam used to form the Bonham, TX 75418 65-acre lake to the boathouse and park headquarters. Visit the CCC-constructed Latitude: 33.546727 picnic area first, which houses my favorite Longitude: -96.144758 footbridge in all of Texas. Online reservations (903) 583-5022 Entrance Fees Adult Day Use: $4 Daily Child 12 and Under: Free Visit park website PHOTO BY CHASE FOUNTAIN / TPWD CHASE BY PHOTO FOUNTAIN � MORE � DAVIS MOUNTAINS STATE PARK PARKS TO VIEW CCC WORK Map and directions Indian Lodge, the pueblo-style hotel in PO Box 1707 Davis Mountains State Park, reflects the Fort Davis, TX 79734 history and culture of the region. -

Heel and Toe 2013/2014 Number 19
HEEL AND TOE ONLINE The official organ of the Victorian Race Walking Club 2013/2014 Number 19 4 February 2014 VRWC Preferred Supplier of Shoes, clothes and sporting accessories. Address: RUNNERS WORLD, 598 High Street, East Kew, Victoria (Melways 45 G4) Telephone: 03 9817 3503 Hours : Monday to Friday: 9:30am to 5:30pm Saturday: 9:00am to 3:00pm Website: http://www.runnersworld.com.aU Facebook: http://www.facebook.com/pages/Runners-World/235649459888840 TIM'S WALKER OF THE WEEK There were plenty of good performances at the Australian 20km Summer Championship meet in Hobart on Sunday but none better than those of Jemima Montag and Jesse Osborne who won the Junior 10km events so it was an easy task to decide on them as joint winners of this week's Walker of the Week. • 15 year old Jemima Montag was dominant in the Junior Women's race, going straight to the lead and passing through the 5km mark in just over 22 mins for a big 5km PB. She did understandably slow from there on but her finishing time of 47:00 was a 2:22 PB. It is a shame that she is too young for this year's World Junior Champs but she is eligible to contest the World Cup and, like Jesse, is an automatic selection as winner of the trial. • 19 year old Jesse also strode straight to the lead in the Junior Men's race and was on target for a sub-41 at the 6km mark before fading slightly in the warming morning conditions. -

XII IAPO Manual of Pediatric Otorhinolaryngology
IAPO Interamerican Association of Pediatric Otorhinolaryngology XII IAPO Manual of Pediatric Otorhinolaryngology Editors TANIA SIH ALBERTO CHINSKI ROLAND EAVEY RICARDO GODINHO Coordinator TANIA SIH Copyright © 2013 Interamerican Association of Pediatric Otorhinolaryngology (IAPO) This book contains information obtained from authentic and highly regarded sources. A wide variety of references or recommended readings are listed. The editors cannot assume responsibility for the validity of all materials or for the consequences of their use. No part of this book may be reprinted, reproduced, transmitted, or utilized in any form by any electronic, mechanical, or other means, now known or hereafter invented, including photocopying, microfilming, and recording, or in any information storage or retrieval system, without written permission from the editor/coordinator. Tania Sih 306 Mato Grosso Street, suite 1510 São Paulo, SP 01239-040 Brazil Fax: (55-11) 2114-6512 E-mail: [email protected] Alberto Chinski Charcas, 2777- 1A 1425 Buenos Aires Argentina Fax: (54-11) 4821-1272 E-mail: [email protected] Roland D. Eavey Bill Wilkerson Center 1215, 21st Ave. South. Medical Center East South Tower, suite 6310 Nashville, TN 37232 FAX : (615) 936-0704 E-mail: [email protected] Ricardo Godinho Rua Dr. Chassim, 208 Sete Lagoas, MG 35700-018 Brazil Fax: (55-31) 3772-2121 E-mail: [email protected] THE DEPOSIT ESTABLISHED IN BRAZIL Graphic Project: Larissa Zimbres De Carvalho ISBN International Standard Book Number: 978-85-60209-03-3 (Hardcover) Published in 2013 by Editora e Gráfica Vida & Consciência Rua Agostinho Gomes, 2312 São Paulo, SP, 04206-001, Brazil Phone: (55-11) 3577 3201 E-mail: [email protected] IAPO´s Board of Directors Ellen Friedman (USA) Officers 2012-2014 Elsa C. -

A Molecular Phylogeny and Classification of the Eleusininae with a New Genus, Micrachne (Poaceae: Chloridoideae: Cynodonteae)
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/271851457 A molecular phylogeny and classification of the Eleusininae with a new genus, Micrachne (Poaceae: Chloridoideae: Cynodonteae) Article in Taxon · June 2015 DOI: 10.12705/643.5 CITATIONS READS 17 379 3 authors, including: Paul M. Peterson Konstantin Romaschenko Smithsonian Institution M.G. Kholodny Institute of Botany 446 PUBLICATIONS 2,908 CITATIONS 94 PUBLICATIONS 1,057 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: TRINIUS: Trinius, Carl Bernhard (1778-1844) Literature & Herbarium View project Revisions of Leptochloa (Poaceae) sensu lato View project All content following this page was uploaded by Konstantin Romaschenko on 16 July 2015. The user has requested enhancement of the downloaded file. TAXON 64 (3) • June 2015: 445–467 Peterson & al. • Phylogeny and classification of the Eleusininae A molecular phylogeny and classification of the Eleusininae with a new genus, Micrachne (Poaceae: Chloridoideae: Cynodonteae) Paul M. Peterson,1 Konstantin Romaschenko1,2 & Yolanda Herrera Arrieta3 1 Smithsonian Institution, Department of Botany, National Museum of Natural History, Washington D.C. 20013-7012, U.S.A. 2 M.G. Kholodny Institute of Botany, National Academy of Sciences, Kiev 01601, Ukraine 3 Instituto Politécnico Nacional, CIIDIR Unidad Durango-COFAA, Durango, C.P. 34220, Mexico Author for correspondence: Paul M. Peterson, [email protected] ORCID: PMP, http://orcid.org/0000000194055528; KR, http://orcid.org/0000000272484193 DOI http://dx.doi.org/10.12705/643.5 Abstract The subtribe Eleusininae (Poaceae: Chloridoideae: Cynodonteae) is a diverse group containing about 212 species in 31 genera found primarily in low latitudes in Africa, Asia, Australia, and the Americas, and the classification among these genera and species is poorly understood. -
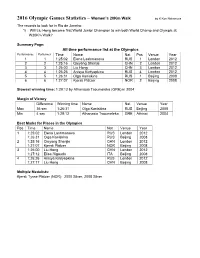
2016 Olympic Games Statistics
2016 Olympic Games Statistics – Women’s 20Km Walk by K Ken Nakamura The records to look for in Rio de Janeiro: 1) Will Liu Hong become first World Junior Champion to win both World Champ and Olympic at W20Km Walk? Summary Page: All time performance list at the Olympics Performance Performer Time Name Nat Pos Venue Year 1 1 1:25:02 Elena Lashmanova RUS 1 London 2012 2 2 1:25:16 Qieyang Shenjie CHN 2 London 2012 3 3 1:26:00 Liu Hong CHN 3 London 2012 4 4 1:26:26 Anisya Kirdyapkina RUS 4 London 2012 5 5 1:26:31 Olga Kaniskina RUS 1 Beijing 2008 6 6 1:27:07 Kjersti Plätzer NOR 2 Beijing 2008 Slowest winning time: 1:29:12 by Athanasia Tsoumeleka (GRE) in 2004 Margin of Victory Difference Winning time Name Nat Venue Year Max 36 sec 1:26:31 Olga Kaniskina RUS Beijing 2008 Min 4 sec 1:29:12 Athanasia Tsoumeleka GRE Athinai 2004 Best Marks for Places in the Olympics Pos Time Name Nat Venue Year 1 1:25:02 Elena Lashmanova RUS London 2012 1:26:31 Olga Kaniskina RUS Beijing 2008 2 1:25:16 Qieyang Shenjie CHN London 2012 1:27:07 Kjersti Plätzer NOR Beijing 2008 3 1:26:00 Liu Hong CHN London 2012 1:27:12 Elisa Rigaudo ITA Beijing 2008 4 1:26:26 Anisya Kirdyapkina RUS London 2012 1:27:17 Liu Hong CHN Beijing 2008 Multiple Medalists: Kjersti Tysse Plätzer (NOR): 2000 Silver, 2008 Silver All time performance list at the Olympics Performance Performer Time Name Nat Pos Venue Year 1 1 1:25:02 Elena Lashmanova RUS 1 London 2012 2 2 1:25:16 Qieyang Shenjie CHN 2 London 2012 3 3 1:26:00 Liu Hong CHN 3 London 2012 4 4 1:26:26 Anisya Kirdyapkina RUS 4 London -

Where to Go... CAMPING
Issue 1 - November 2017 Where to go... CAMPING --A Guide to the Outdoors Outdoor Adventures Department Alamo Area Council, BSA www.AlamoAreaBSA.org For Council Facility Reservations, contact: Matthew Rodriguez (210)740-3549 or [email protected] The purpose of this guide is to provide units with information that will be useful in planing their camping programs. This document has been compiled from informa- tion furnished by the various Boy Scout Councils in Texas as well as from various other sources. The document is intended to be used as a guide. No claim is made as to the accuracy of the data printed herin. Most campsites require that reservations be made in advance, and at that time you should check to see that there has been no change since the publication of this document. Units using any camp should be prepared to follow the outdoor code for the dura- tion of their visit -- it is a sure way of being asked to return. Corrections, additions, and/or changes should be sent to [email protected]. We hope this guide will be of use to you and your unit. Happy Camping! THE SCOUT LAW A Scout is TRUSTWORTHY. A true Scout chooses to keep his promises and awlays tells the truth. It should become part of his conduct so people can always trust what he has to say. A Scout is LOYAL. A Scout is always loyal and true to his friends and family as well as his Scout lead- ers, school, nation, and world community. A Scout is HELPFUL. -

GRAPHIE by Cornelia D. Niles with INTRODUCTION and BOTANICAL
A BIBLIOGRAPHIC STUDY OF BEAUVOIS' AGROSTO- • GRAPHIE By Cornelia D. Niles WITH INTRODUCTION AND BOTANICAL NOTES By Aones Chase nrntODTJCTiON The Essai d?une Nouvelle Agrostographie ; ou Nouveaux Genres des Graminees; avec figures representant les Oaracteres de tous les Genres, by A. M. F. J. Palisot de Beauvois, published in 1812, is, from the standpoint of the nomenclature of grasses, a very important work, its importance being due principally to its innumerable errors, less so because of its scientific value. In this small volume 69 new genera are proposed and some 640 new species, new binomials, and new names are published. Of the 69 genera proposed 31 are to-day recognized as valid, and of the 640 names about 61 are commonly accepted. There is probably not a grass flora of any considerable region anywhere in the world that does not contain some of Beauvois' names. Many of the new names are made in such haphazard fashion that they are incorrectly listed in the Index Kewensis. There are, besides, a number of misspelled names that have found their way into botanical literature. The inaccuracies are so numerous and the cita- tions so incomplete that only a trained bibliographer* could solve the many puzzles presented. Cornelia D. Niles in connection with her work on the bibliography of grasses, maintained in the form of a card catalogue in the Grass Herbarium, worked out the basis in literature of each of these new names. The botanical problems involved, the interpretation of descriptions and figures, were worked out by Agnes Chase, who is also respon- sible for the translation and summaries from the Advertisement, Introduction, and Principles.