Pure Alexia Is a Well-Documented Syndrome Characterized By
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Phonological Activation in Pure Alexia
Cognitive Neuropsychology ISSN: 0264-3294 (Print) 1464-0627 (Online) Journal homepage: http://www.tandfonline.com/loi/pcgn20 Phonological Activation in Pure Alexia Marie Montant & Marlene Behrmann To cite this article: Marie Montant & Marlene Behrmann (2001) Phonological Activation in Pure Alexia, Cognitive Neuropsychology, 18:8, 697-727, DOI: 10.1080/02643290143000042 To link to this article: http://dx.doi.org/10.1080/02643290143000042 Published online: 09 Sep 2010. Submit your article to this journal Article views: 47 View related articles Citing articles: 15 View citing articles Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=pcgn20 Download by: [Carnegie Mellon University] Date: 13 May 2016, At: 08:41 COGNITIVE NEUROPSYCHOLOGY, 2001, 18 (8), 697–727 PHONOLOGICAL ACTIVATION IN PURE ALEXIA Marie Montant CNRS, Marseille, France and Carnegie Mellon University, Pittsburgh, USA Marlene Behrmann Carnegie Mellon University and Center for the Neural Basis of Cognition, Pittsburgh, USA Pure alexia is a reading impairment in which patients appear to read letter-by-letter. This disorder is typically accounted for in terms of a peripheral deficit that occurs early on in the reading system, prior to the activation of orthographic word representations. The peripheral interpretation of pure alexia has recently been challenged by the phonological deficit hypothesis, which claims that a postlexical discon- nection between orthographic and phonological information contributes to or is responsible for the dis- order. Because this hypothesis was mainly supported by data from a single patient (IH), who also has surface dyslexia, the present study re-examined this hypothesis with another pure alexic patient (EL). -
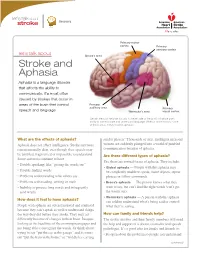
Stroke and Aphasia Aphasia Is a Language Disorder That Affects the Ability to Communicate
Recovery Primary motor cortex Primary sensory cortex let’s talk about Broca’s area Stroke and Aphasia Aphasia is a language disorder that affects the ability to communicate. It’s most often caused by strokes that occur in areas of the brain that control Primary auditory area Primary speech and language. Wernicke’s area visual cortex Certain areas of the brain (usually in the left side of the brain) influence one’s ability to communicate and understand language. When a stroke occurs in one of these areas, it may result in aphasia. What are the effects of aphasia? sender plissen.” Thousands of alert, intelligent men and Aphasia does not affect intelligence. Stroke survivors women are suddenly plunged into a world of jumbled remain mentally alert, even though their speech may communication because of aphasia. be jumbled, fragmented or impossible to understand. Are there different types of aphasia? Some survivors continue to have: Yes, there are several forms of aphasia. They include: • Trouble speaking, like “getting the words out” • Global aphasia — People with this aphasia may • Trouble finding words be completely unable to speak, name objects, repeat • Problems understanding what others say phrases or follow commands. • Problems with reading, writing or math • Broca’s aphasia — The person knows what they • Inability to process long words and infrequently want to say, but can’t find the right words (can’t get used words the words out). • Wernicke’s aphasia — A person with this aphasia How does it feel to have aphasia? can seldom understand what’s being said or control People with aphasia are often frustrated and confused what they’re saying. -

Abadie's Sign Abadie's Sign Is the Absence Or Diminution of Pain Sensation When Exerting Deep Pressure on the Achilles Tendo
A.qxd 9/29/05 04:02 PM Page 1 A Abadie’s Sign Abadie’s sign is the absence or diminution of pain sensation when exerting deep pressure on the Achilles tendon by squeezing. This is a frequent finding in the tabes dorsalis variant of neurosyphilis (i.e., with dorsal column disease). Cross References Argyll Robertson pupil Abdominal Paradox - see PARADOXICAL BREATHING Abdominal Reflexes Both superficial and deep abdominal reflexes are described, of which the superficial (cutaneous) reflexes are the more commonly tested in clinical practice. A wooden stick or pin is used to scratch the abdomi- nal wall, from the flank to the midline, parallel to the line of the der- matomal strips, in upper (supraumbilical), middle (umbilical), and lower (infraumbilical) areas. The maneuver is best performed at the end of expiration when the abdominal muscles are relaxed, since the reflexes may be lost with muscle tensing; to avoid this, patients should lie supine with their arms by their sides. Superficial abdominal reflexes are lost in a number of circum- stances: normal old age obesity after abdominal surgery after multiple pregnancies in acute abdominal disorders (Rosenbach’s sign). However, absence of all superficial abdominal reflexes may be of localizing value for corticospinal pathway damage (upper motor neu- rone lesions) above T6. Lesions at or below T10 lead to selective loss of the lower reflexes with the upper and middle reflexes intact, in which case Beevor’s sign may also be present. All abdominal reflexes are preserved with lesions below T12. Abdominal reflexes are said to be lost early in multiple sclerosis, but late in motor neurone disease, an observation of possible clinical use, particularly when differentiating the primary lateral sclerosis vari- ant of motor neurone disease from multiple sclerosis. -
• Classifications of Aphasia Expressive Vs. Receptive Fluent Vs
12/7/2018 APHASIA Aphasia is an acquired communication disorder that impairs a person’s ability to process LANGUAGE, but DOES NOT AFFECT intelligence. Aphasia impairs the ability to speak and understand others. -National Aphasia Association LANGUAGE Language is a system of communication that uses symbolism. K L U $ + M – Phonemes: perceptually distinct unit of sounds Words: sounds combined & given meaning Sentences: combination of syntax (rules) and semantics (meaning). • CLASSIFICATIONS OF APHASIA EXPRESSIVE VS. RECEPTIVE FLUENT VS. NON- FLUENT 1 12/7/2018 -NATIONAL APHASIA ASSOCIATION -COURTESY OF MY-MS.ORG MCA DISTRIBUTION -SLIDESHARE.NET 2 12/7/2018 BROCA’S APHASIA * short utterances * limited vocabulary * halting, effortful speech *mild comprehension deficits Lesion * Inferior frontal gyrus Choose Sentence Speech Coordinate Speak Idea Words Structure Sounds Articulate Pragmatics Muscles Fluently (Semantics) (Syntax) (Phonology) SAMPLE OF BROCA’S THERAPY FROM TACTUS THERAPY 3 12/7/2018 WERNICKE’S APHASIA • Comprehension is poor (auditory & reading) • Fluent, intact prosody • Logorrhea, press of speech • Neologisms, Paraphasias • Lack of awareness Lesion Temporo-Parietal, Posterior section of the superior temporal gyrus near the auditory cortex Auditory Preparation Attach Input Perception Recognition Phonological For Meaning Analysis Output WERNICKE’S APHASIA FROM TACTUS THERAPY 4 12/7/2018 GLOBAL APHASIA * severe language deficit * responds to personally relevant language * responds to non-verbal cues * some automatic speech Lesion -

Number Reading in Pure Alexiaâ
Neuropsychologia 49 (2011) 2283–2298 Contents lists available at ScienceDirect Neuropsychologia jo urnal homepage: www.elsevier.com/locate/neuropsychologia Reviews and perspectives Number reading in pure alexia—A review a,∗ b Randi Starrfelt , Marlene Behrmann a Center for Visual Cognition, Department of Psychology, Copenhagen University, O. Farimagsgade 2A, DK-1353 Copenhagen K, Denmark b Department of Psychology, Carnegie Mellon University, Pittsburgh, PA, USA a r t i c l e i n f o a b s t r a c t Article history: It is commonly assumed that number reading can be intact in patients with pure alexia, and that this Received 25 October 2010 dissociation between letter/word recognition and number reading strongly constrains theories of visual Received in revised form 31 March 2011 word processing. A truly selective deficit in letter/word processing would strongly support the hypothesis Accepted 22 April 2011 that there is a specialized system or area dedicated to the processing of written words. To date, however, Available online 4 May 2011 there has not been a systematic review of studies investigating number reading in pure alexia and so the status of this assumed dissociation is unclear. We review the literature on pure alexia from 1892 to Keywords: 2010, and find no well-documented classical dissociation between intact number reading and impaired Pure alexia letter identification in a patient with pure alexia. A few studies report strong dissociations, with number Alexia without agraphia reading less impaired than letter reading, but when we apply rigorous statistical criteria to evaluate Letter-by-letter reading Visual recognition these dissociations, the difference in performance across domains is not statistically significant. -

Journal of Neurological Disorders DOI: 10.4172/2329-6895.1000309 ISSN: 2329-6895
olog eur ica N l D f i o s l o a r n d r e u r s o J Lee, et al., J Neurol Disord 2016, 4:7 Journal of Neurological Disorders DOI: 10.4172/2329-6895.1000309 ISSN: 2329-6895 Case Report Open Access Two Cases with Cerebral Infarction in the Left Middle Frontal Lobe Presented as Gerstmann's Syndrome Eun-Ju Lee, Hye-Young Shin, Young Noh, Ki-Hyung Park, Hyeon-Mi Park, Yeong-Bae Lee, Dong-Jin Shin, Young Hee Sung and Dong Hoon Shin* Department of Neurology, Gil Hospital, Gachon University Gil Medical Center, Incheon, South Korea *Corresponding author: Dong Hoon Shin, Department of Neurology, Gil Hospital, Gachon University Gil Medical Center, South Korea, Tel: +82-32-460-3346; Fax: +83-32-460-3344; E-mail: [email protected] Rec date: Oct 08, 2016, Acc date: Oct 18, 2016, Pub date: Oct 22, 2016 Copyright: © 2016 Lee, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Gerstmann's syndrome is a neuropsychological disorder characterized by four symptoms, namely, acalculia, finger agnosia, left-right disorientation, and agraphia suggesting the presence of a lesion in the inferior parietal lobule of the dominant hemisphere, especially at the angular gyrus. Several descriptions of Gerstmann's syndrome have been reported in associated with a lesion to the left frontal lobe, but none of these reports fulfilled the full tetrad of diagnostic criteria. -

26 Aphasia, Memory Loss, Hemispatial Neglect, Frontal Syndromes and Other Cerebral Disorders - - 8/4/17 12:21 PM )
1 Aphasia, Memory Loss, 26 Hemispatial Neglect, Frontal Syndromes and Other Cerebral Disorders M.-Marsel Mesulam CHAPTER The cerebral cortex of the human brain contains ~20 billion neurons spread over an area of 2.5 m2. The primary sensory and motor areas constitute 10% of the cerebral cortex. The rest is subsumed by modality- 26 selective, heteromodal, paralimbic, and limbic areas collectively known as the association cortex (Fig. 26-1). The association cortex mediates the Aphasia, Memory Hemispatial Neglect, Frontal Syndromes and Other Cerebral Disorders Loss, integrative processes that subserve cognition, emotion, and comport- ment. A systematic testing of these mental functions is necessary for the effective clinical assessment of the association cortex and its dis- eases. According to current thinking, there are no centers for “hearing words,” “perceiving space,” or “storing memories.” Cognitive and behavioral functions (domains) are coordinated by intersecting large-s- cale neural networks that contain interconnected cortical and subcortical components. Five anatomically defined large-scale networks are most relevant to clinical practice: (1) a perisylvian network for language, (2) a parietofrontal network for spatial orientation, (3) an occipitotemporal network for face and object recognition, (4) a limbic network for explicit episodic memory, and (5) a prefrontal network for the executive con- trol of cognition and comportment. Investigations based on functional imaging have also identified a default mode network, which becomes activated when the person is not engaged in a specific task requiring attention to external events. The clinical consequences of damage to this network are not yet fully defined. THE LEFT PERISYLVIAN NETWORK FOR LANGUAGE AND APHASIAS The production and comprehension of words and sentences is depen- FIGURE 26-1 Lateral (top) and medial (bottom) views of the cerebral dent on the integrity of a distributed network located along the peri- hemispheres. -
THE SYNDROMES of the ARTERIES of the BRAIN AND, SPINAL CORD Part II by LESLIE G
I19 Postgrad Med J: first published as 10.1136/pgmj.29.329.119 on 1 March 1953. Downloaded from - N/ THE SYNDROMES OF THE ARTERIES OF THE BRAIN AND, SPINAL CORD Part II By LESLIE G. KILOH, M.D., M.R.C.P., D.P.M. First Assistant in the Joint Department of Psychological Medicine, Royal Victoria Infirmary and University of Durham The Vertebral Artery (See also Cabot, I937; Pines and Gilensky, Each vertebral artery enters the foramen 1930.) magnum in front of the roots of the hypoglossal nerve, inclines forwards and medially to the The Posterior Inferior Cerebellar Artery anterior aspect of the medulla oblongata and unites The posterior inferior cerebellar artery arises with its fellow at the lower border of the pons to from the vertebral artery at the level of the lower form the basilar artery. border of the inferior olive and winds round the The posterior inferior cerebellar and the medulla oblongata between the roots of the hypo- Protected by copyright. anterior spinal arteries are its principal branches glossal nerve. It passes rostrally behind the root- and it sometimes gives off the posterior spinal lets of the vagus and glossopharyngeal nerves to artery. A few small branches are supplied directly the lower border of the pons, bends backwards and to the medulla oblongata. These are in line below caudally along the inferolateral boundary of the with similar branches of the anterior spinal artery fourth ventricle and finally turns laterally into the and above with the paramedian branches of the vallecula. basilar artery. Branches: From the trunk of the artery, In some cases of apparently typical throm- twigs enter the lateral aspect of the medulla bosis of the posterior inferior cerebellar artery, oblongata and supply the region bounded ventrally post-mortem examination has demonstrated oc- by the inferior olive and medially by the hypo- clusion of the entire vertebral artery (e.g., Diggle glossal nucleus-including the nucleus ambiguus, and Stcpford, 1935). -

Acquired Alexia Is a Reading Disorder Caused by Neurological Damage
Abstract The primary goal of the present study was to investigate the efficacy of a multisensory reading approach (Lindamood Phoneme Sequencing Program) for the treatment of clients diagnosed with acquired alexia. All three participants improved their decoding skills as an effect of the LiPS program but with distinguishable characteristics believed to relate to their type of acquired alexia. The participant with surface alexia demonstrated the greatest learning curve as compared to the participants with deep or phonological alexia. All three participants showed a positive effect on cognitive-communicative abilities other than reading. Findings will be related to the connectionist approach to reading. Full Text Acquired alexia is a reading disorder caused by neurological damage and is usually the result of small, left-hemisphere, inferior parietal lobe lesions involving the angular gyrus. It is often associated with aphasia and there appears to be some relationship between the severity and nature of aphasic auditory comprehension problems and the severity and nature of alexia. The variables effecting comprehension include word frequency, part of speech, emotionality, personal relevancy, syntactic complexity and length and degree of inference required for interpretation. Individuals with acquired alexia can be classified into four groups: deep alexia, surface alexia, phonological alexia and pure alexia. Error patterns, as they relate to semantics, orthographic length and word frequency distinguish the types of acquired alexia. Some have suggested that phonological alexia is on the continuum of deep alexia (Friedman1). Several approaches have been implemented to facilitate rehabilitation of reading skills. The Multiple Oral Reading (MOR) approach utilized repetition of oral reading to facilitate whole word recognition (Beeson2) whereas the Cross Modality Cueing approach combined kinesthetic and visual information to access the lexicon (Seki3). -

Definition of Stroke/Brain Attack
Definition of Stroke/Brain Attack Stroke II: • A syndrome caused by disruption in the flow of Diagnosis, Evaluation, and Prevention blood to part of the brain due to either: – occlusion of a blood vessel • ischemic stroke Lenore N. Joseph, MD – rupture of a blood vessel Neurology Service Chief, McGuire VAMC • hemorrhagic stroke Assistant Professor of Neurology • The interruption in blood flow deprives the brain VCU Health System of nutrients and oxygen resulting in injury to cells Medical College of Virginia in the affected vascular territory of the brain 1 2 Stroke: The Problem Stroke: The Problem • Third leading cause of death in US • Among 6 month or longer survivors: – after heart disease and cancer – 48% have a hemiparesis • 740,000 new strokes each year – 22% cannot walk • 4.5 million stroke survivors – 24-53% report complete or partial • Leading cause of disability in adults in US dependence for activities • $45.5 billion per year in the USA – 12-18% are aphasic • 1 of 6 Americans will be affected – 32% are clinically depressed – only 10% fully recover 3 4 Symptoms of Brain Attack: Symptoms of Brain Attack: Teach your patients! Teach your patients! • Sudden weakness, paralysis, or numbness of: • Sudden unexplained dizziness –face – especially when associated with other – arm and the leg on one or both sides of the neurologic symptoms body – unsteadiness • Sudden loss of speech, or difficulty speaking or – sudden falls understanding speech • Sudden severe headache and/or loss of • Sudden dimness or loss of vision consciousness – -

Aphasia Accessible Information Guidelines
stroke.org.uk Accessible Information Guidelines Making information accessible for people with aphasia We believe in life after stroke. That’s why we support stroke survivors to make the best recovery they can. It’s why we campaign for better stroke care. And it’s why we fund research into new treatments and ways of preventing stroke. We’re here for you. If you’d like to know more please get in touch. Stroke Helpline: 0303 3033 100 Website: stroke.org.uk Email: [email protected] Textphone: 18001 0303 3033 100 © Stroke Association, July 2012 ISBN 978-0-901548-66-5 All rights reserved. No part of this publication may be reproduced or transmitted, in any form or by any means, electronic, photocopying or otherwise without prior permission of the publishers. NHS workers and teachers may make photocopies for education purposes only, provided that no charge or profit is made for any course or event for which they are used. Published by the Stroke Association Stroke Association House 240 City Road London EC1V 2PR stroke.org.uk Stroke Association is a Company Limited by Guarantee, registered in England and Wales (No 61274). Registered office: Stroke Association House, 240 City Road, London EC1V 2PR. Registered as a Charity in England and Wales (No 211015) and in Scotland (SC037789). Also registered in Northern Ireland (XT33805) Isle of Man (No 945) and Jersey (NPO 369). 2 Stroke Association Contents Authors and acknowledgements 4 Introduction 5 The Five Steps 9 Step 1: A short message 10 Step 2: Clear sentences 11 Step 3: Easy words 14 Step 4: Good layout 15 Step 5: Make a set 21 Checklist 23 Word-processing advice 24 Further Reading 28 Notes 30 Stroke Association is a Company Limited by Guarantee, registered in England and Wales (No 61274). -

DG Case Study
! 1 History DG was a 63-year-old right-handed man who experienced a left hemisphere ischemic stroke in April 2011. Neurology notes from his hospital admission stated that he presented with new onset alexia without agraphia, and anomia. No visual field defects or other neurologic impairments were found. DG’s medical history included atrial fibrillation and hypertension. An MRI shortly after his hospital admission revealed a small acute infarction with hemorrhagic conversion in the inferior left temporal lobe, involving the posterior fusiform gyrus and parahippocampal gyrus. Neurology notes at discharge interpreted the MRI findings as likely due to a venous infarction of the vein of Labbe. DG was a warehouse supervisor but had not worked since his stroke. He had a BA in History and described himself as an avid reader. He was single and lived alone. DG was seen in our outpatient clinic 4 months after his stroke. As part of his application for disability, a neuropsychologist evaluated him 1 month after his stroke. A summary of this evaluation stated that DG had alexia without dysgraphia, mild anomia, and mild memory impairment. When DG came to our clinic, his primary complaint was slow reading, with poor comprehension. He also acknowledged occasional word finding and memory difficulties. Assessment Methods/Tests & Results General Language Assessment and Cognitive Screening DG’s speech was fluent and without any noticeable word finding difficulties or syntactic/ morphological errors. The Comprehensive Aphasia Test (CAT; Swinburn, Porter, & Howard, 2005) was administered to gain an overall assessment of DG’s language abilities. His mean T- ! 2 score for the CAT’s eight language modality scores was 61.3, just below the cut-off of 62.8 used to identify individuals with aphasia.