Diarrhea and Malabsorption
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Antibiotic-Associated Diarrhea: Candidate Organisms Other Than Clostridium Difficile
The Korean Journal of Internal Medicine : 23:9-15, 2008 Antibiotic-Associated Diarrhea: Candidate Organisms other than Clostridium Difficile Hyun Joo Song, M.D.1, Ki-Nam Shim, M.D.1, Sung-Ae Jung, M.D.1, Hee Jung Choi, M.D.1, Mi Ae Lee, M.D.2, Kum Hei Ryu, M.D.1, Seong-Eun Kim, M.D.1 and Kwon Yoo, M.D.1 Department of Internal Medicine1 and Laboratory Medicine2, Ewha Medical Research Institute, College of Medicine, Ewha Womans University, Seoul, Korea Background/Aims : The direct toxic effects of antibiotics on the intestine can alter digestive functions and cause pathogenic bacterial overgrowth leading to antibiotic-associated diarrhea (AAD). Clostridium difficile (C. difficile) is widely known to be responsible for 10~20% of AAD cases. However, Klebsiella oxytoca, Clostridium perfringens, Staphylococcus aureus, and Candida species might also contribute to AAD. Methods : We prospectively analyzed the organisms in stool and colon tissue cultures with a C. difficile toxin A assay in patients with AAD between May and December 2005. In addition, we performed the C. difficile toxin A assays using an enzyme-linked fluorescent assay technique. Patients were enrolled who had diarrhea with more than three stools per day for at least 2 days after the initiation of antibiotic treatment for up to 6~8 weeks after antibiotic discontinuation. Results : Among 38 patients (mean age 59±18 years, M:F=18:20), the organism isolation rates were 28.9% (11/38) for stool culture, 18.4% (7/38) for colon tissue cultures and 13.2% (5/38) for the C. -

Nutritional Disturbances in Crohn's Disease ANTHONY D
Postgrad Med J: first published as 10.1136/pgmj.59.697.690 on 1 November 1983. Downloaded from Postgraduate Medical Journal (November 1983) 59, 690-697 Nutritional disturbances in Crohn's disease ANTHONY D. HARRIES RICHARD V. HEATLEY* M.A., M.R.C.P. M.D., M.R.C.P. Department of Gastroenterology, University Hospital of Wales, Cardiffand *Department ofMedicine, St James's University Hospital, Leeds LS9 7TF Summary deficiency in the same patient. The most important A wide range of nutritional disturbances may be causes of malnutrition are probably reduced food found in patients with Crohn's disease. As more intake, active inflammation and enteric loss of sophisticated tests become available to measure nutrients (Dawson, 1972). vitamin and trace element deficiencies, so these are being recognized as complications ofCrohn's disease. TABLE 1. Pathogenesis of malnutrition It is important to recognize nutritional deficiencies at an early stage and initiate appropriate treatment. Reduced food intake Anorexia Otherwise many patients, experiencing what can be a Fear of eating from abdominal pain chronic and debilitating illness, may suffer unneces- Active inflammation Mechanisms unknown Protected by copyright. sarily from the consequences of deprivation of vital Enteric loss of nutrients Exudation from intestinal mucosa nutrients. Interrupted entero-hepatic circulation Malabsorption Loss of absorptive surface from disease, resection or by-pass KEY WORDS: growth disturbance, Crohn's disease, anaemia, vitamin deficiency. Stagnant loop syndrome from strictures, fistulae or surgically created blind loops Introduction Miscellaneous Rapid gastrointestinal transit Effects of medical therapy Crohn's disease is a chronic inflammatory condi- Effects of parenteral nutrition tion ofunknown aetiology that may affect any part of without trace element supplements the gastrointestinal tract from mouth to anus. -

Does Your Patient Have Bile Acid Malabsorption?
NUTRITION ISSUES IN GASTROENTEROLOGY, SERIES #198 NUTRITION ISSUES IN GASTROENTEROLOGY, SERIES #198 Carol Rees Parrish, MS, RDN, Series Editor Does Your Patient Have Bile Acid Malabsorption? John K. DiBaise Bile acid malabsorption is a common but underrecognized cause of chronic watery diarrhea, resulting in an incorrect diagnosis in many patients and interfering and delaying proper treatment. In this review, the synthesis, enterohepatic circulation, and function of bile acids are briefly reviewed followed by a discussion of bile acid malabsorption. Diagnostic and treatment options are also provided. INTRODUCTION n 1967, diarrhea caused by bile acids was We will first describe bile acid synthesis and first recognized and described as cholerhetic enterohepatic circulation, followed by a discussion (‘promoting bile secretion by the liver’) of disorders causing bile acid malabsorption I 1 enteropathy. Despite more than 50 years since (BAM) including their diagnosis and treatment. the initial report, bile acid diarrhea remains an underrecognized and underappreciated cause of Bile Acid Synthesis chronic diarrhea. One report found that only 6% Bile acids are produced in the liver as end products of of British gastroenterologists investigate for bile cholesterol metabolism. Bile acid synthesis occurs acid malabsorption (BAM) as part of the first-line by two pathways: the classical (neutral) pathway testing in patients with chronic diarrhea, while 61% via microsomal cholesterol 7α-hydroxylase consider the diagnosis only in selected patients (CYP7A1), or the alternative (acidic) pathway via or not at all.2 As a consequence, many patients mitochondrial sterol 27-hydroxylase (CYP27A1). are diagnosed with other causes of diarrhea or The classical pathway, which is responsible for are considered to have irritable bowel syndrome 90-95% of bile acid synthesis in humans, begins (IBS) or functional diarrhea by exclusion, thereby with 7α-hydroxylation of cholesterol catalyzed interfering with and delaying proper treatment. -

Hepatitis A, B, and C: Learn the Differences
Hepatitis A, B, and C: Learn the Differences Hepatitis A Hepatitis B Hepatitis C caused by the hepatitis A virus (HAV) caused by the hepatitis B virus (HBV) caused by the hepatitis C virus (HCV) HAV is found in the feces (poop) of people with hepa- HBV is found in blood and certain body fluids. The virus is spread HCV is found in blood and certain body fluids. The titis A and is usually spread by close personal contact when blood or body fluid from an infected person enters the body virus is spread when blood or body fluid from an HCV- (including sex or living in the same household). It of a person who is not immune. HBV is spread through having infected person enters another person’s body. HCV can also be spread by eating food or drinking water unprotected sex with an infected person, sharing needles or is spread through sharing needles or “works” when contaminated with HAV. “works” when shooting drugs, exposure to needlesticks or sharps shooting drugs, through exposure to needlesticks on the job, or from an infected mother to her baby during birth. or sharps on the job, or sometimes from an infected How is it spread? Exposure to infected blood in ANY situation can be a risk for mother to her baby during birth. It is possible to trans- transmission. mit HCV during sex, but it is not common. • People who wish to be protected from HAV infection • All infants, children, and teens ages 0 through 18 years There is no vaccine to prevent HCV. -

Bowel Function Anatomy
BOWEL FUNCTION ANATOMY Most of America gives little thought to bowel control. However, bowel control is actually a complex process involving the coordination of many different muscles and nerves. The bowel is considered to be a part of the digestive or gastrointestinal system. It is designed to help the body absorb nutrients and fluids from the foods we eat and drink. After taking out everything the body needs, the bowel then expels the leftover waste. The beginning of the bowel is the small intestine, sometimes referred to as the small bowel. This is where the useful nutrients are absorbed from what you eat. The small bowel delivers the waste to the colon, or large bowel. The colon is a 5-6 foot long muscular tube that delivers stool to the rectum. As the stool moves through the colon, the fluids are removed and absorbed into the body. The consistency of the stool is dependent upon many things, including how long the stool sits in the colon, how much of the water has been absorbed from the waste, and the amount of fiber and fluids in your diet. Stool consistency can vary from hard lumps to mushy to very loose, watery stool. The best and easiest consistency of stool is soft, like toothpaste; this consistency may be attained by adding fiber to your diet. Fiber helps move waste through the colon because it is indigestible by the human body. In other words, fiber adds ‘bulk’ to the stool. It is important to eat a diet high in fiber, however, most Americans lack fiber in their diet. -

Malabsorption and Exocrine Pancreatic Insuffiecienty (Pi)
MALABSORPTION AND EXOCRINE PANCREATIC INSUFFIECIENTY (PI) Pancreatic Insufficiency is a condition in which a person does not have enough enzymes and bicarbonate being delivered from the pancreas to the intestine for digestion. This causes mal- absorption of nutrients, failure to gain weight and grow, weight loss, vitamin and mineral deficiency, and gastrointestinal symptoms. Most people with CF have mal-absorption due to PI. Onset usually occurs in the first one to two years of life, often in early infancy, but can start at anytime. Symptoms of mal-absorption -Change in number of stools -Large, bulky stools -Stools may be bulky and soft -Greasy, oily or floating stools, oil in toilet water -Stools may smell worse than usual or normal -Rectal prolapse -Mal-absorption of calorie providing nutrients and poor weight gain or weight loss Fat …………………………………………….9 calories/gram Protein………………………………..…….4 calories/gram Complex Carbohydrate ……………..4 calories/gram -Results in poor weight gain, weight loss, poor growth, decreased immune function and decreased lung health. -Mal-absorption of FAT SOLUBLE VITAMIN and deficiency: Vitamin A, Vitamin D, Vitamin E, Vitamin K -Mineral deficiencies: Calcium, Zinc, Sodium, Chloride Learn more about vitamins and minerals at: http://www.cff.org/treatments/Therapies/Nutrition/Vitamins/ Tests to Diagnose PI and Mal-absorption -72 hr fecal fat test -Pancreatic Fecal Elastase Treatment of PI and Mal-absorption Pancreatic Enzyme Replacement Therapy (PERT) Pancreatic enzymes are taken with each meal, snack, breast feed, bottle , and drink that contains fat protein and or complex carbohydrate. Antacid and acid blocking medicines can be added to make enzymes work better Fat Soluble Vitamin Supplementation with special supplements made for mal-absorption are prescribed Each enzyme company offers programs that provide free nutritional support and/or CF therapy support High Calorie, high protein diet Even with PERT, not all calories and nutrients from food are absorbed as expected and calories and nutrients are lost and need replacement. -

Digestive Health Center Nutrition Services Nutrition Guidelines for Chronic Pancreatitis Patient Education
Digestive Health Center Nutrition Services Nutrition Guidelines for Chronic Pancreatitis Patient Education The pancreas is an organ that: Produces pancreatic enzymes to help digest (break down) food in the small intestine for absorption Makes hormones (such as insulin) to help control blood sugars Chronic pancreatitis is ongoing inflammation of the pancreas. Symptoms can be worse after eating. Symptoms include: Abdominal pain Nausea Vomiting Weight loss Fatty stools (stools may also float and/or have a foul odor) Malabsorption of nutrients can occur from poor digestion of food (due to reduced pancreatic enzyme activity), which will result in nutrients passing into the stools. This is seen especially with fat and fat soluble vitamins (A, D, E) as digestion of fat is highly dependent on pancreatic enzymes. In some cases, diabetes can develop if the pancreas is not able to make enough insulin to help control blood sugars, so blood sugars stay high. Nutritional Guidelines Follow a low fat diet, which for chronic pancreatitis is often restricted to 50 grams of fat, but could also range between 30-50 grams of fat depending on tolerance. If you have diabetes, eat recommended serving sizes of low fat carbohydrates to help control blood sugars (low fat/non fat dairy, fruits, vegetables, whole grains, beans, lentils etc). Information on serving sizes is available. Take pancreatic enzymes as prescribed by your doctor to treat malabsorption. Take the enzymes before each meal and snack. They will not work if taken at the end of the meal. 1 Low Fat Diet Tips Eat 4-6 small meals throughout the day Spread out your fat intake throughout the day Use butter, margarine and cooking oils sparingly Bake, grill, roast and/or steam foods. -

Viral Gastroenteritis Backgrounder
Viral Gastroenteritis Backgrounder Viral Gastroenteritis What is Viral Gastroenteritis? Viral gastroenteritis is a stomach illness (including diarrhea and vomiting) in people that is caused by a virus. It is commonly found throughout North America and Europe, and though it can occur year-round, this illness is most often reported in winter. These viruses can also be easily spread in situations of communal living. Viruses are very different from bacteria and parasites. Viruses are much smaller, are not affected by treatment with antibiotics. What are the symptoms of viral gastroenteritis illness? The symptoms of gastroenteritis illness usually include nausea, vomiting, diarrhea, and some stomach cramping. Sometimes people also have a low-grade fever, chills, headache, muscle aches, and a general sense of tiredness. The illness often begins suddenly, and the infected person may feel very sick. The illness is usually brief, with symptoms usually lasting only about 1 or 2 days. In general, children experience more vomiting than adults. Most people with this type of illness have both vomiting and diarrhea. How serious is viral gastroenteritis? Though this type of illness is usually not serious, some people may feel very sick and vomit or have watery diarrhea many times a day. Most people get better within 1 or 2 days, and have no long-term health effects related to their illness; however, if the ill person is unable to drink enough liquids to replace the liquids they lost because of vomiting and diarrhea, they can become dehydrated and may need special medical attention. This problem with dehydration is usually only seen among the very young, the elderly, and persons with weakened immune systems. -
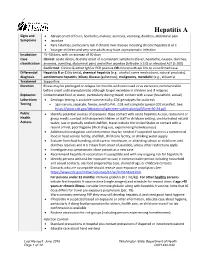
Hepatitis a Reporting Guideline
Hepatitis A Signs and • Abrupt onset of fever, headache, malaise, anorexia, vomiting, diarrhea, abdominal pain Symptoms • Jaundice • Rare fatalities, particularly risk if chronic liver disease including chronic hepatitis B or C • Younger children and very rare adults may have asymptomatic infection Incubation 15–50 days, with an average of 30 days Case Clinical: acute illness, discrete onset of a consistent symptoms (fever, headache, nausea, diarrhea, classification anorexia, vomiting, abdominal pain) and either jaundice (bilirubin ≥ 3.0) or elevated ALT (≥ 200) Confirmed: Clinical & either IgM or PCR positive OR clinical with epi link to a confirmed case Differential Hepatitis B or C (do tests), chemical hepatitis (e.g., alcohol, some medications, natural products), diagnosis autoimmune hepatitis, biliary disease (gallstones), malignancy, metabolic (e.g., Wilson’s) Treatment Supportive Duration Illness may be prolonged or relapse for months with continued virus excretion; communicable before onset until asymptomatic although longer excretion in children and if relapses Exposures Contaminated food or water, particularly during travel; contact with a case (household, sexual) Laboratory • Serologic testing is available commercially; CDC genotypes for outbreak Testing • Spin serum, separate, freeze, send to PHL. CDE will complete special CDC manifest. See: https://www.cdc.gov/laboratory/specimen-submission/pdf/form-50-34.pdf. Public • Identify potential sources of exposure: close contact with acute hepatitis A case, restaurant or Health -

GASTROINTESTINAL COMPLAINT Nausea, Vomiting, Or Diarrhea (For Abdominal Pain – Refer to SO-501) I
DESCHUTES COUNTY ADULT JAIL SO-559 L. Shane Nelson, Sheriff Standing Order Facility Provider: October 17, 2018 STANDING ORDER GASTROINTESTINAL COMPLAINT Nausea, Vomiting, or Diarrhea (for Abdominal Pain – refer to SO-501) I. ASSESSMENT a. History i. Onset and duration ii. Frequency of vomiting, nausea, or diarrhea iii. Blood in stool or black stools? Blood in emesis or coffee-ground appearance? If yes, refer to SO-510 iv. Medications taken – do they help? v. Do they have abdominal pain? If yes, refer to SO-501 Abdominal Pain. vi. Do they have other symptoms – dysuria, urinary frequency, urinary urgency, urinary incontinence, vaginal/penile discharge, hematuria, fever, chills, flank pain, abdominal/pelvic pain in females or testicular pain in males, vaginal or penile lesions/sores? (if yes to any of the above – refer to Dysuria SO-522) vii. LMP in female inmates – if unknown, obtain HCG viii. History of substance abuse? Are they withdrawing? Refer to appropriate SO based on substance history and withdrawal concerns. ix. History of IBS or other known medical causes of chronic diarrhea, nausea, or vomiting? Have prescriptions been used for this in the past? x. History of abdominal surgeries? xi. Recent exposure to others with same symptoms? b. Exam i. Obtain Vital signs, including temperature ii. If complaints of dizziness or lightheadedness with standing, obtain orthostatic VS. iii. Is there jaundice present? iv. Are there signs of dehydration – tachycardia, tachypnea, lethargy, changes in mental status, dry mucous membranes, pale skin color, decreased skin turgor? v. Are you concerned for an Acute Gastroenteritis? Supersedes: March 20, 2018 Review Date: October 2020 Total Pages: 3 1 SO-559 October 17, 2018 Symptoms Exam Viruses cause 75-90% of acute gastroenteritis here in the US. -

Acute Diarrhea in Adults WENDY BARR, MD, MPH, MSCE, and ANDREW SMITH, MD Lawrence Family Medicine Residency, Lawrence, Massachusetts
Acute Diarrhea in Adults WENDY BARR, MD, MPH, MSCE, and ANDREW SMITH, MD Lawrence Family Medicine Residency, Lawrence, Massachusetts Acute diarrhea in adults is a common problem encountered by family physicians. The most common etiology is viral gastroenteritis, a self-limited disease. Increases in travel, comorbidities, and foodborne illness lead to more bacteria- related cases of acute diarrhea. A history and physical examination evaluating for risk factors and signs of inflammatory diarrhea and/or severe dehydration can direct any needed testing and treatment. Most patients do not require labora- tory workup, and routine stool cultures are not recommended. Treatment focuses on preventing and treating dehydra- tion. Diagnostic investigation should be reserved for patients with severe dehydration or illness, persistent fever, bloody stool, or immunosuppression, and for cases of suspected nosocomial infection or outbreak. Oral rehydration therapy with early refeeding is the preferred treatment for dehydration. Antimotility agents should be avoided in patients with bloody diarrhea, but loperamide/simethicone may improve symptoms in patients with watery diarrhea. Probiotic use may shorten the duration of illness. When used appropriately, antibiotics are effective in the treatment of shigellosis, campylobacteriosis, Clostridium difficile,traveler’s diarrhea, and protozoal infections. Prevention of acute diarrhea is promoted through adequate hand washing, safe food preparation, access to clean water, and vaccinations. (Am Fam Physician. 2014;89(3):180-189. Copyright © 2014 American Academy of Family Physicians.) CME This clinical content cute diarrhea is defined as stool with compares noninflammatory and inflamma- conforms to AAFP criteria increased water content, volume, or tory acute infectious diarrhea.7,8 for continuing medical education (CME). -

Nutrition Management in the Adult Patient with Crohn's Disease
Review Article: Nutrition management in the adult patient with Crohn’s disease Nutrition management in the adult patient with Crohn’s disease Basson A, MS RD(USA)(SA) Lecturer and Hospital Student Internship Supervisor, University of the Western Cape Correspondence to: Abigail Basson, email: [email protected] Keywords: inflammatory bowel disease, nutrition therapy, Crohn’s disease Abstract Malnutrition, nutrient deficiencies and osteoporosis are common in patients with Crohn’s disease, regardless of disease activity. While the role of diet in the pathogenesis of the disease remains inconclusive, upon diagnosis, nutrition therapy plays an integral role in patient care. Successful nutrition intervention involves appropriate nutritional assessment, supplemental nutrition and individualised counselling and support. Peer reviewed. (Submitted: 2012-05-08. Accepted: 2012-11-04.) © SAJCN S Afr J Clin Nutr 2012;25(4):164-172 Introduction Low-fibre, high-fat and high-sugar intakes have been implicated as some of the environmental triggers in disease development, Crohn’s disease (CD) is a chronic and recurrent immune-mediated although the role of a pre-illness diet in the pathogenesis of CD inflammatory disorder of the gastrointestinal tract.1,2 Typically, pa- remains inconclusive.16-18 However, upon diagnosis, nutrition therapy tients suffer from chronic intestinal inflammation that follows a plays an integral role in patient care, regardless of disea se activity. relapse-remitting pattern, as well as from a variety of complications 3,4 (Table I) that may or may not involve the gut. Disease activity can Malnutrition be classified by the Crohn’s Disease Activity Index5 (CDAI) (Table II), and usually, treatment includes various combinations of corticoste- Weight loss, low body mass index (BMI) and nutrient deficiencies roid, anti-inflammatory (aminosalicylates), immune-modulating or have been well documented in patients with CD, especially during biological therapy.4 While the exact cause of CD is not known, it is active disease.