Structure and Synthesis of Alkenes Alkenes (Olefins) Are Hydrocarbons Which Have Carbon–Carbon Double Bonds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cinnamoyl Esters of Lesquerella and Castor Oil: Novel Sunscreen Active Ingredients David L
Cinnamoyl Esters of Lesquerella and Castor Oil: Novel Sunscreen Active Ingredients David L. Compton*, Joseph A. Laszlo, and Terry A. Isbell New Crops and Processing Technology Research Unit, USDA, ARS, NCAUR, Peoria, Illinois 61604 ABSTRACT: Lesquerella and castor oils were esterified with querolin (2). Therefore, LO in essence contains two moles of cinnamic acid (CA) and 4-methoxycinnamic acid (MCA). Esteri- hydroxy functionality per mole of triglyceride (TG). fication of the hydroxy oils reached 85% completion with CA The hydroxy functionality of the FA of the TG can be ex- and 50% conversion with MCA. The hydroxy oils were esteri- ploited by esterification to form estolides. The majority of re- fied at 200°C under a nitrogen atmosphere within a sealed sys- search has focused on the estolides of hydroxy FA and TG tem. Unreacted CA and MCA were removed from the reaction formed with oleic acid. The esterification of hydroxy TG and mixtures by sublimation at 100°C under vacuum. The resultant free lesquerolic acid with oleic acid using a cobalt catalyst (4) methoxycinnamic oils possessed a broader, more blue-shifted or a lipase catalyst (5) has been reported. Also, the acid-cat- UV absorbance, 250 to 345 nm with a λmax of 305 nm, com- pared with the cinnamic oils, which absorbed from 260 to 315 alyzed formation of estolides from CO and LO with oleic acid has been patented (6). Recently, a detailed study of the effect nm, λmax of 270 nm. The methoxycinnamic oils provide better UV-B absorption and thus are better candidates to be used as of oleic acid concentration and temperature on catalyst-free sunscreen active ingredients. -

Transcription 12.02.15
Lecture 12A • 02/15/12 We’re going to continue our discussion of conjugation. If we go back and talk about electrons and how they act like waves, [there’s] something known as the particle in a box. You’ve got some kind of box where imagine that the walls are infinitely tall. It’s a one-dimensional system, where the electron, all it can do, is go left and right between the two walls of the box. The electron’s a wave; it can’t exist outside the box, so whatever function, whatever wave we use to describe the electron, has to have a value of zero at one end of the box and zero at the other end of the box. Graphically, what do the solutions look like? We have something like this, where we’re talking about energy potential; it’s infinite at either end of the box, and zero in between. We have an electron that’s bouncing around inside of it. An electron’s a wave, and there’s function that describes that wave. The only way it can fit in this box and physically make sense is if the wave starts and stops and the ends of the box. It turns out that – long, long, long story short – one of the solutions for the Schrödinger equation is just a sine function – actually, it’s part of an exponential version of a sine function. It’s something, at least, we can draw a pretty picture of. That’s why you’ll start with this problem in a discussion of quantum mechanics, because it is solvable, it is only on in dimension, and it’s more humanly possible to discuss. -

Aromaticity Sem- Ii
AROMATICITY SEM- II In 1931, German chemist and physicist Sir Erich Hückel proposed a theory to help determine if a planar ring molecule would have aromatic properties .This is a very popular and useful rule to identify aromaticity in monocyclic conjugated compound. According to which a planar monocyclic conjugated system having ( 4n +2) delocalised (where, n = 0, 1, 2, .....) electrons are known as aromatic compound . For example: Benzene, Naphthalene, Furan, Pyrrole etc. Criteria for Aromaticity 1) The molecule is cyclic (a ring of atoms) 2) The molecule is planar (all atoms in the molecule lie in the same plane) 3) The molecule is fully conjugated (p orbitals at every atom in the ring) 4) The molecule has 4n+2 π electrons (n=0 or any positive integer Why 4n+2π Electrons? According to Hückel's Molecular Orbital Theory, a compound is particularly stable if all of its bonding molecular orbitals are filled with paired electrons. - This is true of aromatic compounds, meaning they are quite stable. - With aromatic compounds, 2 electrons fill the lowest energy molecular orbital, and 4 electrons fill each subsequent energy level (the number of subsequent energy levels is denoted by n), leaving all bonding orbitals filled and no anti-bonding orbitals occupied. This gives a total of 4n+2π electrons. - As for example: Benzene has 6π electrons. Its first 2π electrons fill the lowest energy orbital, and it has 4π electrons remaining. These 4 fill in the orbitals of the succeeding energy level. The criteria for Antiaromaticity are as follows: 1) The molecule must be cyclic and completely conjugated 2) The molecule must be planar. -

Unit One Part 2: Naming and Functional Groups
gjr-–- 1 Unit One Part 2: naming and functional groups • To write and interpret IUPAC names for small, simple molecules • Identify some common functional groups found in organic molecules O CH3 H3C N H N O N N O H3C S N O N H3C viagra™ (trade name) sildenafil (trivial name) 5-(2-ethoxy-5-(4-methylpiperazin-1-ylsulfonyl)phenyl)-1-methyl-3-propyl-1H- pyrazolo[4,3-d] pyrimidin-7(6H)-one dr gareth rowlands; [email protected]; science tower a4.12 http://www.massey.ac.nz/~gjrowlan gjr-–- 2 Systematic (IUPAC) naming PREFIX PARENT SUFFIX substituents / minor number of C principal functional functional groups AND multiple bond group index • Comprises of three main parts • Note: multiple bond index is always incorporated in parent section No. Carbons Root No. Carbons Root Multiple-bond 1 meth 6 hex Bond index 2 eth 7 hept C–C an(e) 3 prop 8 oct C=C en(e) 4 but 9 non C≡C yn(e) 5 pent 10 dec gjr-–- 3 Systematic (IUPAC) naming: functional groups Functional group Structure Suffix Prefix General form O –oic acid acid –carboxylic acid carboxy R-COOH R OH O O –oic anhydride anhydride –carboxylic anhydride R-C(O)OC(O)-R R O R O –oyl chloride acyl chloride -carbonyl chloride chlorocarbonyl R-COCl R Cl O –oate ester –carboxylate alkoxycarbonyl R-COOR R OR O –amide amide –carboxamide carbamoyl R-CONH2 R NH2 nitrile R N –nitrile cyano R-C≡N O –al aldehyde –carbaldehyde oxo R-CHO R H O ketone –one oxo R-CO-R R R alcohol R OH –ol hydroxy R-OH amine R NH2 –amine amino R-NH2 O ether R R –ether alkoxy R-O-R alkyl bromide bromo R-Br (alkyl halide) R Br (halo) (R-X) gjr-–- 4 Nomenclature rules 1. -

Polymer Exemption Guidance Manual POLYMER EXEMPTION GUIDANCE MANUAL
United States Office of Pollution EPA 744-B-97-001 Environmental Protection Prevention and Toxics June 1997 Agency (7406) Polymer Exemption Guidance Manual POLYMER EXEMPTION GUIDANCE MANUAL 5/22/97 A technical manual to accompany, but not supersede the "Premanufacture Notification Exemptions; Revisions of Exemptions for Polymers; Final Rule" found at 40 CFR Part 723, (60) FR 16316-16336, published Wednesday, March 29, 1995 Environmental Protection Agency Office of Pollution Prevention and Toxics 401 M St., SW., Washington, DC 20460-0001 Copies of this document are available through the TSCA Assistance Information Service at (202) 554-1404 or by faxing requests to (202) 554-5603. TABLE OF CONTENTS LIST OF EQUATIONS............................ ii LIST OF FIGURES............................. ii LIST OF TABLES ............................. ii 1. INTRODUCTION ............................ 1 2. HISTORY............................... 2 3. DEFINITIONS............................. 3 4. ELIGIBILITY REQUIREMENTS ...................... 4 4.1. MEETING THE DEFINITION OF A POLYMER AT 40 CFR §723.250(b)... 5 4.2. SUBSTANCES EXCLUDED FROM THE EXEMPTION AT 40 CFR §723.250(d) . 7 4.2.1. EXCLUSIONS FOR CATIONIC AND POTENTIALLY CATIONIC POLYMERS ....................... 8 4.2.1.1. CATIONIC POLYMERS NOT EXCLUDED FROM EXEMPTION 8 4.2.2. EXCLUSIONS FOR ELEMENTAL CRITERIA........... 9 4.2.3. EXCLUSIONS FOR DEGRADABLE OR UNSTABLE POLYMERS .... 9 4.2.4. EXCLUSIONS BY REACTANTS................ 9 4.2.5. EXCLUSIONS FOR WATER-ABSORBING POLYMERS........ 10 4.3. CATEGORIES WHICH ARE NO LONGER EXCLUDED FROM EXEMPTION .... 10 4.4. MEETING EXEMPTION CRITERIA AT 40 CFR §723.250(e) ....... 10 4.4.1. THE (e)(1) EXEMPTION CRITERIA............. 10 4.4.1.1. LOW-CONCERN FUNCTIONAL GROUPS AND THE (e)(1) EXEMPTION................. -

8.3 Bonding Theories >
8.3 Bonding Theories > Chapter 8 Covalent Bonding 8.1 Molecular Compounds 8.2 The Nature of Covalent Bonding 8.3 Bonding Theories 8.4 Polar Bonds and Molecules 1 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals Molecular Orbitals How are atomic and molecular orbitals related? 2 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals • The model you have been using for covalent bonding assumes the orbitals are those of the individual atoms. • There is a quantum mechanical model of bonding, however, that describes the electrons in molecules using orbitals that exist only for groupings of atoms. 3 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals • When two atoms combine, this model assumes that their atomic orbitals overlap to produce molecular orbitals, or orbitals that apply to the entire molecule. 4 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals Just as an atomic orbital belongs to a particular atom, a molecular orbital belongs to a molecule as a whole. • A molecular orbital that can be occupied by two electrons of a covalent bond is called a bonding orbital. 5 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals Sigma Bonds When two atomic orbitals combine to form a molecular orbital that is symmetrical around the axis connecting two atomic nuclei, a sigma bond is formed. • Its symbol is the Greek letter sigma (σ). -

Studies on the Chemistry of Paclitaxel
STUDIES ON THE CHEMISTRY OF PACLITAXEL Haiqing Yuan Dissertation submitted to the Faculty of the Virginia Polytechnic Institute and State University in the partial fulfillment of the requirement for the degree of Doctor of Philosophy in Chemistry Dr. David G. I. Kingston, Chair Dr. Michael Calter Dr. Neal Castagnoli, Jr. Dr. Richard Gandour Dr. Larry Taylor August 11, 1998 Blacksburg, Virginia Keywords: Paclitaxel, Taxol®, synthesis, analog, SAR Copyright 1998, Haiqing Yuan STUDIES ON THE CHEMISTRY OF PACLITAXEL HAIQING YUAN (ABSTRACT) Paclitaxel is a natural occurring diterpene alkaloid originally isolated from the bark of Taxus brevifolia. It is now one of the most important chemotherapeutic agents for clinical treatment of ovarian and breast cancers. Recent clinical trials have also shown paclitaxel’s potential for the treatment of non-small-cell lung cancer, head and neck cancer, and other types of cancers. While tremendous chemical research efforts have been made in the past years, which established the fundamental structure-activity relationships of the paclitaxel molecule, and provided analogs for biochemical studies to elucidate the precise mechanism of action and for the development of second-generation agents, many areas remain to be explored. In continuation of our efforts in the structure-activity relationships study of A- norpaclitaxel, five new analogs modified at the C-1 substituent and analogs with expanded B-ring or contracted C-ring have now been prepared. Preliminary biological studies indicated that the volume rather than functionality at the C-1 position plays a role in determining the anticancer activity by controlling the relative position of the tetracyclic ring system, which in turn controls the positions of the most critical functionalities such as the C-2 benzoyl, the C-4 acetate, and the C-13 side chain. -

The Arene–Alkene Photocycloaddition
The arene–alkene photocycloaddition Ursula Streit and Christian G. Bochet* Review Open Access Address: Beilstein J. Org. Chem. 2011, 7, 525–542. Department of Chemistry, University of Fribourg, Chemin du Musée 9, doi:10.3762/bjoc.7.61 CH-1700 Fribourg, Switzerland Received: 07 January 2011 Email: Accepted: 23 March 2011 Ursula Streit - [email protected]; Christian G. Bochet* - Published: 28 April 2011 [email protected] This article is part of the Thematic Series "Photocycloadditions and * Corresponding author photorearrangements". Keywords: Guest Editor: A. G. Griesbeck benzene derivatives; cycloadditions; Diels–Alder; photochemistry © 2011 Streit and Bochet; licensee Beilstein-Institut. License and terms: see end of document. Abstract In the presence of an alkene, three different modes of photocycloaddition with benzene derivatives can occur; the [2 + 2] or ortho, the [3 + 2] or meta, and the [4 + 2] or para photocycloaddition. This short review aims to demonstrate the synthetic power of these photocycloadditions. Introduction Photocycloadditions occur in a variety of modes [1]. The best In the presence of an alkene, three different modes of photo- known representatives are undoubtedly the [2 + 2] photocyclo- cycloaddition with benzene derivatives can occur, viz. the addition, forming either cyclobutanes or four-membered hetero- [2 + 2] or ortho, the [3 + 2] or meta, and the [4 + 2] or para cycles (as in the Paternò–Büchi reaction), whilst excited-state photocycloaddition (Scheme 2). The descriptors ortho, meta [4 + 4] cycloadditions can also occur to afford cyclooctadiene and para only indicate the connectivity to the aromatic ring, and compounds. On the other hand, the well-known thermal [4 + 2] do not have any implication with regard to the reaction mecha- cycloaddition (Diels–Alder reaction) is only very rarely nism. -

Organic Chemistry Tests for Hydroxyl Group
Chemistry Organic Chemistry Tests for Hydroxyl Group General Aim Method Identication of aliphatic alcohols through the chemical Detection of the presence of hydroxyl groups in detection of hydroxyl groups. aliphatic alcohols using special chemical tests. Learning Objectives (ILOs) Dene and determine aliphatic alcohols theoretically through their chemical structure. Classify organic compounds containing hydroxyl groups into aliphatic and aromatic. Compare between alcohols and other functional groups in terms of chemical structures, properties and reactions. Identify aliphatic alcohols experimentally. Select the appropriate reagents to dierentiate between alcohols and other organic compounds. Theoretical Background/Context - Aliphatic alcohols are non-aromatic hydrocarbons possessing at least one hydroxyl group within their structure. They can be either cyclic or acyclic compounds. Alcohols are considered to be neutral compounds. Aliphatic Cyclic Alcohol Aliphatic Acyclic Alcohol - Alcohols are also classied to primary, secondary and tertiary alcohols according to the number of carbon atoms attached to the carbon atom linked to the hydroxyl group. First: Preparation of Aliphatic Alcohols - Alcohols can be prepared through some chemical routes such as reduction of the corresponding aldehydes and ketones using some reducing agents such as lithium aluminum hydride or sodium borohydride. They can be also obtained from hydration of the corresponding alkenes. Some primary alcohols can be synthesized through the nucleophilic substitution of corresponding alkyl halides using potassium or sodium hydroxides. - Alcohols can be also obtained through some biological routes such as ethanol and butanol through fermentation processes in presence of glucose. Glucose is obtained from starch hydrolysis in the presence of yeast. 1 www.praxilabs.com Theoretical Background/Context (Cont’) Second: General Properties of Aliphatic Alcohols - Aliphatic alcohols are polar compounds and can form hydrogen bonds easily. -

Aromatic Compounds
OCR Chemistry A Aromatic Compounds Aromatic Compounds Naming Aromatic compounds contain one or more benzene rings (while aliphatic compounds do not contain benzene rings). Another term for a compound containing a benzene ring is arene. The basic benzene ring, C6H6 is commonly represented as a hexagon with a ring inside. You should be aware that there is a hydrogen at each corner although this is not normally shown. N.B. it is OK to use benzene in this form in structural formulae too! Arenes occur naturally in many substances, and are present in coal and crude oil. Aspirin, for example, is an aromatic compound, an arene: HO O O CH 3 O Naming of substances based on benzene follows familiar rules: CH Br NO 3 2 benzene methylbenzene bromobenzene nitrobenzene Numbers are needed to identify the positions of substituents. The carbons around the ring are numbered from 1-6 consecutively and the name which gives the lowest number(s) is chosen: CH3 CH3 Cl Br Cl Br 2-bromomethylbenzene 4-bromomethylbenzene 1,2-dichlorobenzene Cl Cl Cl 1,3,5-trichlorobenzene Page 1 OCR Chemistry A Aromatic Compounds Determining the structure of benzene (historical) 1825 – substance first isolated by Michael Faraday, who also determined its empirical formula as CH 1834 – RFM of 78 and molecular formula of C6H6 determined H H H C C C Much speculation over the structure. Many suggested structures like: C C C H H H 1865 – Kekulé publishes suggestion of a ring with alternating double and single bonds: H H C H C C displayed C C skeletal H C H H This model persisted until 1922, but not all chemists accepted the structure because it failed to explain the chemical and physical properties of benzene fully: if C=C bonds were present as Kekulé proposed, then benzene would react like alkenes. -

Alkenes and Alkynes
02/21/2019 CHAPTER FOUR Alkenes and Alkynes H N O I Cl C O C O Cl F3C C Cl C Cl Efavirenz Haloprogin (antiviral, AIDS therapeutic) (antifungal, antiseptic) Chapter 4 Table of Content * Unsaturated Hydrocarbons * Introduction and hybridization * Alkenes and Alkynes * Benzene and Phenyl groups * Structure of Alkenes, cis‐trans Isomerism * Nomenclature of Alkenes and Alkynes * Configuration cis/trans, and cis/trans Isomerism * Configuration E/Z * Physical Properties of Hydrocarbons * Acid‐Base Reactions of Hydrocarbons * pka and Hybridizations 1 02/21/2019 Unsaturated Hydrocarbons • Unsaturated Hydrocarbon: A hydrocarbon that contains one or more carbon‐carbon double or triple bonds or benzene‐like rings. – Alkene: contains a carbon‐carbon double bond and has the general formula CnH2n. – Alkyne: contains a carbon‐carbon triple bond and has the general formula CnH2n‐2. Introduction Alkenes ● Hydrocarbons containing C=C ● Old name: olefins • Steroids • Hormones • Biochemical regulators 2 02/21/2019 • Alkynes – Hydrocarbons containing C≡C – Common name: acetylenes Unsaturated Hydrocarbons • Arene: benzene and its derivatives (Ch 9) 3 02/21/2019 Benzene and Phenyl Groups • We do not study benzene and its derivatives until Chapter 9. – However, we show structural formulas of compounds containing a phenyl group before that time. – The phenyl group is not reactive under any of the conditions we describe in chapters 5‐8. Structure of Alkenes • The two carbon atoms of a double bond and the four atoms bonded to them lie in a plane, with bond angles of approximately 120°. 4 02/21/2019 Structure of Alkenes • Figure 4.1 According to the orbital overlap model, a double bond consists of one bond formed by overlap of sp2 hybrid orbitals and one bond formed by overlap of parallel 2p orbitals. -

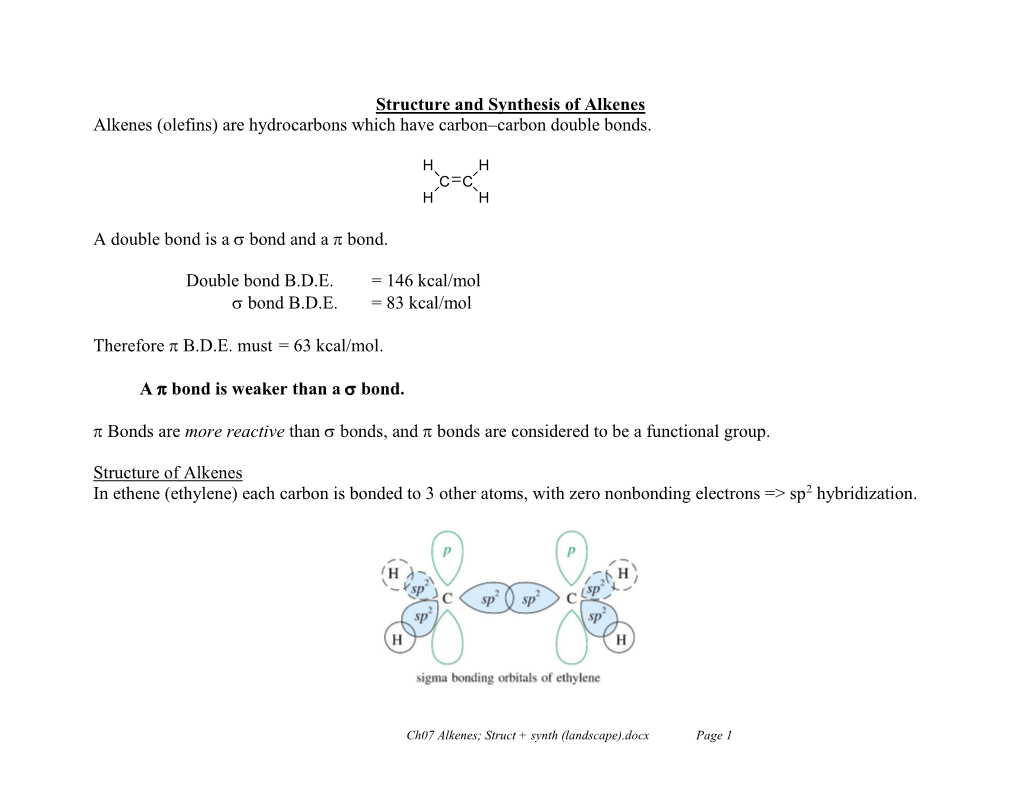
Introduction to Alkenes and Alkynes in an Alkane, All Covalent Bonds
Introduction to Alkenes and Alkynes In an alkane, all covalent bonds between carbon were σ (σ bonds are defined as bonds where the electron density is symmetric about the internuclear axis) In an alkene, however, only three σ bonds are formed from the alkene carbon -the carbon thus adopts an sp2 hybridization Ethene (common name ethylene) has a molecular formula of CH2CH2 Each carbon is sp2 hybridized with a σ bond to two hydrogens and the other carbon Hybridized orbital allows stronger bonds due to more overlap H H C C H H Structure of Ethylene In addition to the σ framework of ethylene, each carbon has an atomic p orbital not used in hybridization The two p orbitals (each with one electron) overlap to form a π bond (p bonds are not symmetric about the internuclear axis) π bonds are not as strong as σ bonds (in ethylene, the σ bond is ~90 Kcal/mol and the π bond is ~66 Kcal/mol) Thus while σ bonds are stable and very few reactions occur with C-C bonds, π bonds are much more reactive and many reactions occur with C=C π bonds Nomenclature of Alkenes August Wilhelm Hofmann’s attempt for systematic hydrocarbon nomenclature (1866) Attempted to use a systematic name by naming all possible structures with 4 carbons Quartane a alkane C4H10 Quartyl C4H9 Quartene e alkene C4H8 Quartenyl C4H7 Quartine i alkine → alkyne C4H6 Quartinyl C4H5 Quartone o C4H4 Quartonyl C4H3 Quartune u C4H2 Quartunyl C4H1 Wanted to use Quart from the Latin for 4 – this method was not embraced and BUT has remained Used English order of vowels, however, to name the groups