Lecture 12: O2 Binding by Myoglobin & Hemoglobin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Myoglobin Expression Under Hypoxic Condtions In
THESIS IN THE FACE OF HYPOXIA: MYOGLOBIN EXPRESSION UNDER HYPOXIC CONDTIONS IN CULTURED WEDDELL SEAL SKELETAL MUSCLE CELLS Submitted by Michael Anthony De Miranda Jr. Department of Biology In partial fulfillment of the requirements For the degree of Master of Science Colorado State University Fort Collins, Colorado Spring 2012 Master’s Committee: Advisor: Shane Kanatous Gregory Florant Scott Earley Copyright by Michael A. De Miranda Jr. 2012 All Rights Reserved ABSTRACT IN THE FACE OF HYPOXIA: MYOGLOBIN EXPRESSION UNDER HYPOXIC CONDITIONS IN CULTURED WEDDELL SEAL SKELETAL MUSCLE CELLS The hallmark adaptation to breath-hold diving in Weddell seals (Leptonychotes weddellii) is enhanced concentrations of myoglobin in their skeletal muscles. Myoglobin is a cytoplasmic hemoprotein that stores oxygen for use in aerobic metabolism throughout the dive duration. In addition, throughout the duration of the dive, Weddell seals rely on oxygen stored in myoglobin to sustain aerobic metabolism in which lipid is the primary contributor of acetyl CoA for the citric acid cycle. Together, enhanced myoglobin concentrations and a lipid-based aerobic metabolism represent some of the unique adaptations to diving found in skeletal muscle of Weddell seals. This thesis presents data that suggests cultured Weddell seal skeletal muscle cells inherently possess adaptations to diving such as increased myoglobin concentrations, and rely on lipids to fuel aerobic metabolism. I developed the optimum culture media for this unique primary cell line based on myoblast confluence, myoblast growth rates, myotube counts, and myotube widths. Once the culture media was established, I then determined the de novo expression of myoglobin under normoxic and hypoxic oxygen conditions and the metabolic profile of the myotubes under each oxygen condition. -

Nucleosome-Mediated Cooperativity Between Transcription Factors
Nucleosome-mediated cooperativity between transcription factors Leonid A. Mirny1 Harvard–MIT Division of Health Sciences and Technology, and Department of Physics, Massachusetts Institute of Technology, Cambridge, MA 02139 Edited* by José N. Onuchic, University of California San Diego, La Jolla, CA, and approved October 12, 2010 (received for review December 3, 2009) Cooperative binding of transcription factors (TFs) to promoters and A B other regulatory regions is essential for precise gene expression. The classical model of cooperativity requires direct interactions between TFs, thus constraining the arrangement of TF sites in reg- ulatory regions. Recent genomic and functional studies, however, demonstrate a great deal of flexibility in such arrangements with variable distances, numbers of sites, and identities of TF sites lo- cated in cis-regulatory regions. Such flexibility is inconsistent with L C N O D T R cooperativity by direct interactions between TFs. Here, we demon- P 0 0 0 0 P O O strate that strong cooperativity among noninteracting TFs can be K K 2 2 NN O O T R achieved by their competition with nucleosomes. We find that the P 1 1 P 1 1 K O O mechanism of nucleosome-mediated cooperativity is analogous to c = O 10 1..10 3 2 2 K cooperativity in another multimolecular complex: hemoglobin. This N N2 O2 T R [P] P 2 2 = 15 P surprising analogy provides deep insights, with parallels between O2 O2 KO … … the heterotropic regulation of hemoglobin (e.g., the Bohr effect) [N ] L = 0 100 1000 N O and the roles of nucleosome-positioning sequences and chromatin [O0] n n T4 R4 modifications in gene expression. -

Pi-Pi Stacking Mediated Cooperative Mechanism for Human Cytochrome P450 3A4
Molecules 2015, 20, 7558-7573; doi:10.3390/molecules20057558 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Article Pi-pi Stacking Mediated Cooperative Mechanism for Human Cytochrome P450 3A4 Botao Fa 1, Shan Cong 2 and Jingfang Wang 1,* 1 Key Laboratory of Systems Biomedicine (Ministry of Education), Shanghai Center for Systems Biomedicine, Shanghai Jiao Tong University, Shanghai 200240, China; E-Mail: [email protected] 2 Department of Bioinformatics and Biostatistics, College of Life Sciences and Biotechnology, Shanghai Jiao Tong University, Shanghai 200240, China; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +86-21-3420-7344 (ext. 123); Fax: +86-21-3420-4348. Academic Editor: Antonio Frontera Received: 13 January 2015 / Accepted: 19 March 2015 / Published: 24 April 2015 Abstract: Human Cytochrome P450 3A4 (CYP3A4) is an important member of the cytochrome P450 superfamily with responsibility for metabolizing ~50% of clinical drugs. Experimental evidence showed that CYP3A4 can adopt multiple substrates in its active site to form a cooperative binding model, accelerating substrate metabolism efficiency. In the current study, we constructed both normal and cooperative binding models of human CYP3A4 with antifungal drug ketoconazoles (KLN). Molecular dynamics simulation and free energy calculation were then carried out to study the cooperative binding mechanism. Our simulation showed that the second KLN in the cooperative binding model had a positive impact on the first one binding in the active site by two significant pi-pi stacking interactions. The first one was formed by Phe215, functioning to position the first KLN in a favorable orientation in the active site for further metabolism reactions. -

Human Physiology an Integrated Approach
Gas Exchange and Transport Gas Exchange in the Lungs and Tissues 18 Lower Alveolar P Decreases Oxygen Uptake O2 Diff usion Problems Cause Hypoxia Gas Solubility Aff ects Diff usion Gas Transport in the Blood Hemoglobin Binds to Oxygen Oxygen Binding Obeys the Law of Mass Action Hemoglobin Transports Most Oxygen to the Tissues P Determines Oxygen-Hb Binding O2 Oxygen Binding Is Expressed As a Percentage Several Factors Aff ect Oxygen-Hb Binding Carbon Dioxide Is Transported in Three Ways Regulation of Ventilation Neurons in the Medulla Control Breathing Carbon Dioxide, Oxygen, and pH Infl uence Ventilation Protective Refl exes Guard the Lungs Higher Brain Centers Aff ect Patterns of Ventilation The successful ascent of Everest without supplementary oxygen is one of the great sagas of the 20th century. — John B. West, Climbing with O’s , NOVA Online (www.pbs.org) Background Basics Exchange epithelia pH and buff ers Law of mass action Cerebrospinal fl uid Simple diff usion Autonomic and somatic motor neurons Structure of the brain stem Red blood cells and Giant liposomes hemoglobin of pulmonary Blood-brain barrier surfactant (40X) From Chapter 18 of Human Physiology: An Integrated Approach, Sixth Edition. Dee Unglaub Silverthorn. Copyright © 2013 by Pearson Education, Inc. All rights reserved. 633 Gas Exchange and Transport he book Into Thin Air by Jon Krakauer chronicles an ill- RUNNING PROBLEM fated trek to the top of Mt. Everest. To reach the summit of Mt. Everest, climbers must pass through the “death zone” T High Altitude located at about 8000 meters (over 26,000 ft ). Of the thousands of people who have attempted the summit, only about 2000 have been In 1981 a group of 20 physiologists, physicians, and successful, and more than 185 have died. -

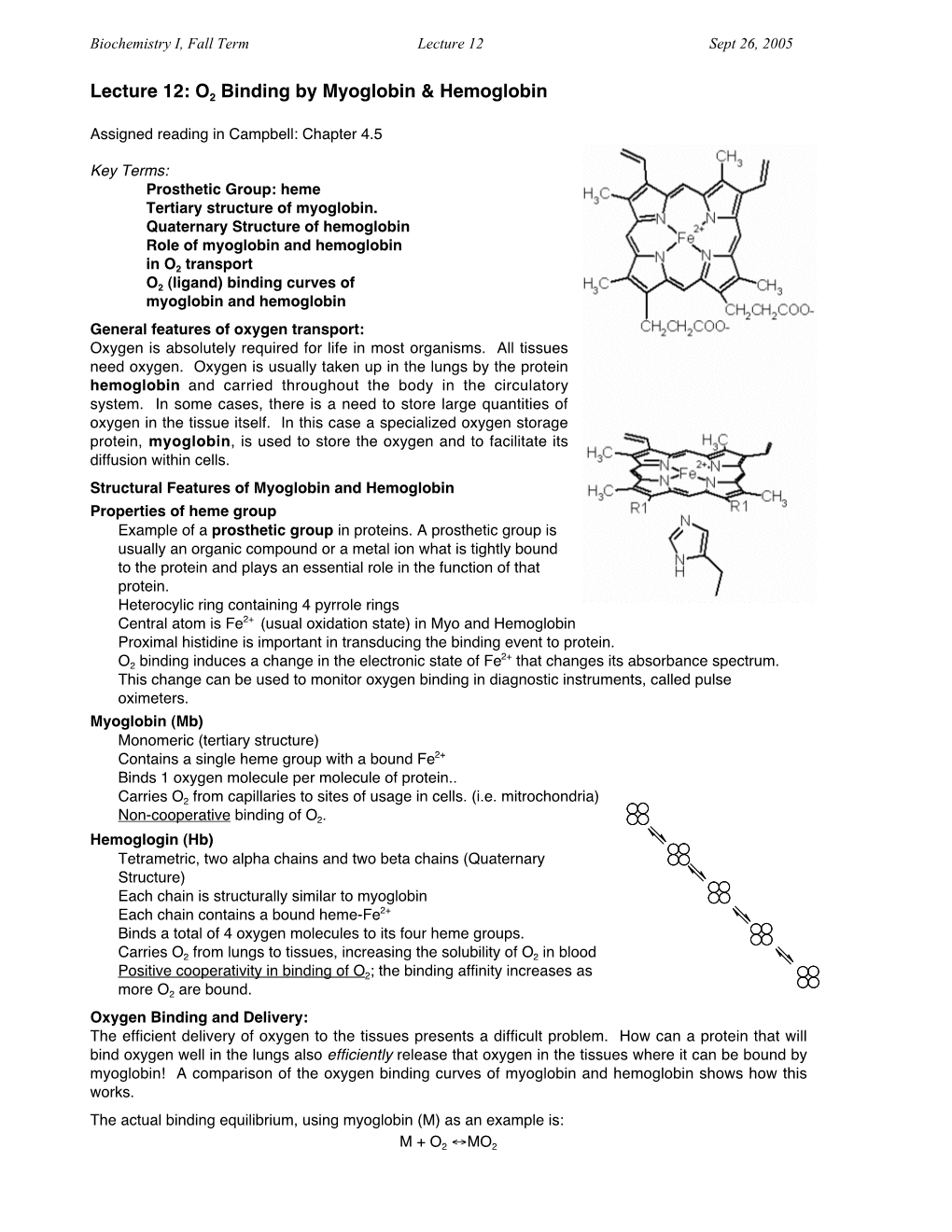
Allosteric Effects and Cooperative Binding
Biochemistry I, Fall Term Lecture 13 Sept 28, 2005 Lecture 13: Allosteric Effects and Cooperative Binding Assigned reading in Campbell: Chapter 4.7, 7.2 Key Terms: • Homotropic Allosteric effects • Heterotropic Allosteric effects • T and R states of cooperative systems • Role of proximal His residue in cooperativity of O2 binding by Hb • Regulation of O2 binding to Hb by bis-phosphoglycerate (BPG) Oxygen Binding to Myoglobin and Hemolobin: Myoglobin binds one O2: Hemoglobin binds four O2: Allosteric Effects and Cooperativity: Allosteric effects occur when the binding properties of a macromolecule change as a consequence of a second ligand binding to the macromolecule and altering its affinity towards the first, or primary, ligand. There need not be a direct connection between the two ligands (i.e. they may bind to opposite sides of the protein, or even to different subunits) • If the two ligands are the same (e.g. oxygen) then this is called a homo-tropic allosteric effect. • If the two ligands are different (e.g. oxygen and BPG), then this is called a hetero-tropic allosteric effect. In the case of macromolecules that have multiple ligand binding sites (e.g. Hb), allosteric effects can generate cooperative behavior. Allosteric effects are important in the regulation of enzymatic reactions. Both allosteric activators (which enhance activity) and allosteric inhibitors (which reduce activity) are utilized to control enzyme reactions. Allosteric effects require the presence of two forms of the macromolecule. One form, usually called the T or tense state, binds the primary ligand (e.g. oxygen) with low affinity. The other form, usually called the R or relaxed state, binds ligand with high affinity. -

Mathematical Toolkit for Quantitative Analysis of Cooperative Binding of Two Or More Ligands to a Substrate Jacob Peacock and James B
Mathematical toolkit for quantitative analysis of cooperative binding of two or more ligands to a substrate Jacob Peacock and James B. Jaynes Dept. of Biochemistry and Molecular Biology, Thomas Jefferson University, Philadelphia PA 19107 United States of America [email protected] Abstract We derive mathematical expressions for quantitative analysis of cooperative binding covering the following cases: 1) a single ligand binds to either two non-equivalent sites, or an arbitrary number of equivalent sites, on a substrate (Fig. 1), and 2) two different ligands bind distinct sites on a substrate (Figs. 2, 3). We show how to analyze "competition experiments" using non-linear regression, where a ligand binds to a single site on a labeled substrate in the presence of increasing amounts of identical but unlabeled competitor substrate, to simultaneously determine the Kd and active ligand concentration (Fig. 4A). We compare the performance of this competition method with the commonly used saturation binding method (Fig. 4B). We also provide methods to analyze such experiments that include a second competitor substrate with non-specific binding sites. We show how to build on results from single-ligand competition experiments to fully characterize cooperative binding in systems with two distinct ligands and binding sites (Fig. 4C and Section 5). We generalize the methodology to more than two cooperating ligands, such as an array of DNA binding proteins (Fig. 6). See Peacock and Jaynes [1] for discussion of the various ways these tools can be used, and results using them. • Visualize characteristics and limitations of Hill plots applied to more realistic binding models than those described by the Hill equation, which implies multiple simultaneous ligand binding. -

Absolute Measurements of Cerebral Perfusion and Oxygenation in Rats with Near-Infrared Spectroscopy Bertan Hallacoglu1, Angelo Sassaroli1, Irwin H
Absolute measurements of cerebral perfusion and oxygenation in rats with near-infrared spectroscopy Bertan Hallacoglu1, Angelo Sassaroli1, Irwin H. Rosenberg2, Aron Troen2 and Sergio Fantini1 Tufts University Department of Biomedical Engineering, Medford, MA (1) and USDA Human Nutrition Research Center on Aging, Boston, MA (2) Brain microvascular pathology is a common finding in Alzheimer’s disease and other dementias. However, Figure 2 – Time traces of [Hb], [HbO2], [HbT], StO2 and SaO2 during the hypoxia and hypercapnia protocols 10 and 20 weeks after Figure 3 – Illustration of the differences in animal groups and the extent to which microvascular abnormalities cause or contribute to cognitive impairment is unclear. the start of folate deficient diet (blue and red lines represent the mean values for each dietary group, whereas, dashed lines indicate changes within each group between weeks 10 and 20 in Dietary vascular risk factors, including poor folate status are potentially modifiable predictors of cognitive the range corresponding to ± one standard error from the mean). A first striking result is the consistency of baseline values across cerebral tissue saturation (StO2) and concentration of impairment among older adults. Folate deficiency in rat impairs cognition and causes cerebral animals within a group (control or folate deficient), and the reproducibility of baseline values measured at weeks 10 and 20. hemoglobin ([HbT]), blood concentration of hemoglobin ([HbT]b), microvascular damage, without concomitant neurodegeneration [1]. We hypothesized that folate Absolute brain total hemoglobin concentration ([HbT]) and tissue oxygen saturation (StO2) are significantly reduced by folate and partial blood volume (Vb/Vt) Eq. (1). FD rats have deficiency might result in functional decrements in cerebral oxygen delivery and vascular reactivity. -

The Effect of Anemia on the Ventilatory Response to Transient and Steady-State Hypoxia
The effect of anemia on the ventilatory response to transient and steady-state hypoxia. T V Santiago, … , N H Edelman, A P Fishman J Clin Invest. 1975;55(2):410-418. https://doi.org/10.1172/JCI107945. Research Article The effects of anemia upon the ventilatory responses to transient and steady-state hypoxia were studied in unanesthetized goats. Responses to transient hypoxia (inhalation of several breaths of nitrogen) were considered to reflect peripheral chemoreceptor and non-chemoreceptor influences of hypoxia upon ventilatory control. In all goats, severe anemia (hemoglobin 3.1-4.8 g/100ml) markedly heightened the responses to transient hypoxia (from a mean of 0.27 to a mean of 0.75 liter/min/percent fall in SaO2). This phenomenon was substantially reversed by alpha-adrenergic blockade (phenoxybenzamine, 5 mg/kg). In contrast, the ventilatory responses to steady-state hypoxia were unaffected by severe anemia. These data suggest that severe anemia enhances the peripheral chemoreceptor-mediated response to hypoxia through a mechanism involving the alpha-adrenergic system. It also appears that a ventilatory depressant effect of hypoxia which is not mediated by the peripheral chemoreceptors is also enhanced by severe anemia, thereby preventing an increase in the steady-state ventilatory response to hypoxia. Finally, experiments involving variation in oxygen affinity of hemoglobin suggested that O2 tension rather than O2 availability in arterial blood is the major determinant of peripheral chemoreceptor activity. Find the latest version: https://jci.me/107945/pdf The Effect of Anemia on the Ventilatory Response to Transient and Steady-State Hypoxia TEODORO V. SANTIAGO, NORMAN H. -

Myoglobin from Equine Skeletal Muscle
Myoglobin from equine skeletal muscle Catalog Number M0630 Storage Temperature –20 C CAS RN 100684-32-0 Precautions and Disclaimer This product is for R&D use only, not for drug, Product Description household, or other uses. Please consult the Safety Molecular mass:1 17.6 kDa Data Sheet for information regarding hazards and safe Extinction coefficient:2 EmM = 12.92 (555 nm) handling practices. pI:3 7.3 (major component) and 6.8 (minor component) Preparation Instructions Myoglobin from horse skeletal muscle is a single chain This protein is soluble in water (10 mg/ml), yielding a heme protein containing 153 amino acid residues. It clear, red brown solution. posesses no disulfide bridges or free -SH groups. Myoglobin contains 8 variously sized right-handed References helical regions, joined by non-ordered or random coil 1. Darbre, P.D. et al., Comparison of the myoglobin of regions. These 8 helices (A, B, C, D, E, F, G, and H) the zebra (Equus burchelli) with that of the horse are folded back on top of one another, and the heme is (Equus cabalus). Biochim. Biophys. Acta, 393(1), situated between helices E and F. The heme is almost 201-204 (1975). totally buried. Only the edge carrying the two 2. Bowen, W.J., The absorption spectra and extinction hydrophylic propionic acid groups is exposed. The coefficients of myoglobin. J. Biol. Chem., 179, 235- heme is held in position by a coordinating complex 245 (1949). between the central Fe(II) atom and 2 histidine residues 3. Radola, B.J., Isoelectric focusing in layers of (on helices E and F, respectively). -

Respiration the Main Function of the Respiratory System Is Gas Exchange
Respiration The main function of the respiratory system is gas exchange. Gas exchange is achieved through a process called respiration, or breathing. The cardiovascular system and the respiratory system work together to accomplish respiration. External Respiration During external respiration, fresh oxygen from outside the body fills the lungs and alveoli, and carbon dioxide is transported from the body tissues to the lungs. For oxygen to reach the body’s tissues and carbon dioxide to leave the body, gas exchange must occur in the alveolar capillary membrane. The alveoli and the capillaries that surround them make up the alveolar (al-VEE-oh-lar) capillary membrane (Figure 9.5 in the textbook). The alveolar capillary membrane is built for gas exchange, as explained by Fick’s Law. Fick’s Law states that the diffusion of oxygen and carbon dioxide between the capillaries and the alveolar sacs is proportional to the surface area (S.A.) of the lungs, the diffusion constant (D) of each gas, and the difference in partial pressure between each capillary and alveolar sac (P1-P2). According to this law, the diffusion of gases is also inversely related to the thickness of the tissues (T) involved. In simpler terms, thin-walled tissues allow for easier gas exchange. Diffusion = Therefore, oxygen easily diffuses across the membrane of the alveolar sacs and into the capillaries. Carbon dioxide passes from the capillaries into the alveolar sacs, where it can be expelled from the body via the lungs (Figure 9.5). How fast does gas exchange occur in the lungs? Faster than you might think. -

Thermodynamic Versus Kinetic Discrimination of Cooperativity of Enzymatic Ligand Binding
Open Access Austin Biochemistry Special Article - Enzyme Kinetics Thermodynamic versus Kinetic Discrimination of Cooperativity of Enzymatic Ligand Binding Das B1, Banerjee K2 and Gangopadhyay G1* 1Department of Chemical Physics, SN Bose National Abstract Centre for Basic Sciences, India In this work, we have introduced thermodynamic measure to characterize the 2Department of Chemistry, A.J.C. Bose College, India nature of cooperativity in terms of the variation of standard free energy change *Corresponding author: Gangopadhyay G, SN Bose with the fraction of ligand-bound sub-units of a protein in equilibrium, treating National Centre for Basic Sciences, Block-JD, Sector-III, the protein-ligand attachment as a stochastic process. The fraction of ligand- Salt Lake, Kolkata-700106, India bound sub-units of cooperative ligand-binding processes are calculated by the formulation of stochastic master equation for both the KNF and MWC Allosteric Received: December 26, 2018; Accepted: May 23, cooperative model. The proposed criteria of this cooperative measurement is 2019; Published: May 30, 2019 valid for all ligand concentrations unlike the traditional kinetic measurement of Hill coefficient at half-saturation point. A Kullback-Leibler distance is also introduced which indicates how much average standard free energy is involved if a non-cooperative system changes to a cooperative one, giving a quantitative synergistic measure of cooperativity as a function of ligand concentration which utilizes the full distribution function beyond the mean and variance. For the validation of our theory to provide a systematic approach to cooperativity, we have considered the experimental result of the cooperative binding of aspartate to the dimeric receptor of Salmonella typhimurium. -

Structural Basis for Regiospecific Midazolam Oxidation by Human Cytochrome P450 3A4
Structural basis for regiospecific midazolam oxidation by human cytochrome P450 3A4 Irina F. Sevrioukovaa,1 and Thomas L. Poulosa,b,c aDepartment of Molecular Biology and Biochemistry, University of California, Irvine, CA 92697-3900; bDepartment of Chemistry, University of California, Irvine, CA 92697-3900; and cDepartment of Pharmaceutical Sciences, University of California, Irvine, CA 92697-3900 Edited by Michael A. Marletta, University of California, Berkeley, CA, and approved November 30, 2016 (received for review September 28, 2016) Human cytochrome P450 3A4 (CYP3A4) is a major hepatic and Here we describe the cocrystal structure of CYP3A4 with the intestinal enzyme that oxidizes more than 60% of administered drug midazolam (MDZ) bound in a productive mode. MDZ therapeutics. Knowledge of how CYP3A4 adjusts and reshapes the (Fig. 1) is the benzodiazepine most frequently used for pro- active site to regioselectively oxidize chemically diverse com- cedural sedation (9) and is a marker substrate for the CYP3A pounds is critical for better understanding structure–function rela- family of enzymes that includes CYP3A4 (10). MDZ is hydrox- tions in this important enzyme, improving the outcomes for drug ylated by CYP3A4 predominantly at the C1 position, whereas metabolism predictions, and developing pharmaceuticals that the 4-OH product accumulates at high substrate concentrations have a decreased ability to undergo metabolism and cause detri- (up to 50% of total product) and inhibits the 1-OH metabolic mental drug–drug interactions. However, there is very limited pathway (11–14). The reaction rate and product distribution structural information on CYP3A4–substrate interactions available strongly depend on the assay conditions and can be modulated by to date.