Lecture 15 Trace Element Modeling Friday, March 11Th, 2005 • Batch
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Role of Trace Minerals in Animal Production
ROLE OF TRACE MINERALS IN ANIMAL PRODUCTION What Do I Need to Know About Trace Minerals for Beef and Dairy Cattle, Horses, Sheep and Goats? Connie K. Larson, Ph.D. Research Nutritionist, Zinpro Corporation Eden Praire, MN 55344 Presented at the 2005 Nutrition Conference sponsored by Department of Animal Science, UT Extension and University Professional and Personal Development The University of Tennessee. Introduction The role of trace minerals in animal production is an area of strong interest for producers, feed manufactures, veterinarians and scientists. Adequate trace mineral intake and absorption is required for a variety of metabolic functions including immune response to pathogenic challenge, reproduction and growth. Mineral supplementation strategies quickly become complex because differences in trace mineral status of all livestock and avian species is critical in order to obtain optimum production in modern animal production systems. Subclinical or marginal deficiencies may be a larger problem than acute mineral deficiency because specific clinical symptoms are not evident to allow the producer to recognize the deficiency; however, animals continue to grow and reproduce but at a reduced rate. As animal trace mineral status declines immunity and enzyme functions are compromised first, followed by a reduction in maximum growth and fertility, and finally normal growth and fertility decrease prior to evidence of clinical deficiency (Figure 1; Fraker, 1983; Wikse 1992). In order to maintain animals in adequate trace mineral status, balanced intake and absorption are necessary. Figure 1. Effect of declining trace mineral status on animal performance Mineral Status Immunity & Enzyme Function Adequate Maximum Growth/Fertility Normal Growth/Fertility Clinical Signs Subclinical Clinical Trace Mineral Function To better understand the role of trace minerals in animal production it is important to recognize that trace elements are functional components of numerous metabolic events. -

Determination of Trace Element Levels in Patients with Burst Fractures
ORIGINAL ARTICLE Determination of trace element levels in patients with burst fractures Shahab Ahmed Salih Gezh, M.D.,1 Abdurrahman Aycan, M.D.,2 Halit Demir, M.D.,1 Cemal Bozlına, M.D.3 1Department of Chemistry, Yüzüncü Yıl University Faculty of Science and Literature, Van-Turkey 2Department of Neurosurgery, Yüzüncü Yıl University Faculty of Medicine, Van-Turkey 3Department of Neurosurgery, Van Training and Research Hospital, Van-Turkey ABSTRACT BACKGROUND: This study aimed to determine trace element levels (Zn, Fe, Mn, Mg, Cu, Cd, Co, and Pb) in patients with burst fractures in Van Province, Turkey. METHODS: The study included a total of 44 participants with no additional pathologies, including 22 patients with burst fractures aged over 18 years who were admitted to the neurosurgery departments at two hospitals between June 15, 2015 and January 20, 2016 and 22 healthy volunteers. Serum samples were obtained from all participants to measure the serum levels of trace and heavy elements, including Mn, Cd, Cu, Pb, Fe, Co and Zn, using atomic absorbance spectrophotometry. RESULTS: The trace element levels of Zn, Mn, Cu, Co, and Mg were significantly lower (p<0.001), whereas those of Fe, Cd, and Pb were significantly higher in the patient group than in the control group. In addition, the levels of Zn, Mn, Cu, Co, and Mg were lower and the levels of Fe, Cd, and Pb were higher in the patient group than in the control group. CONCLUSION: The probability of burst fracture and its causes leading to any injury may be considered as an indicator balance for the concentration of trace elements between the patient group and control group and may also be a risk factor associated with the bone exposed to burst fracture Significant changes in serum levels of Zn, Cd, Mn, Mg, Pb, Fe, Cu and Zn elements can be observed in patients with burst fractures. -

Experimental Partitioning of Rb, Cs, Sr, and Ba Between Alkali Feldspar and Peraluminous Melt
American Mineralogist, Volume 81, pages 719-734,1996 Experimental partitioning of Rb, Cs, Sr, and Ba between alkali feldspar and peraluminous melt JONATHAN IcENHOWER AND DAVID LoNDON School of Geology and Geophysics, University of Oklahoma, 100 East Boyd Street, SEC 810, Norman, Oklahoma 73019, U.S.A. ABSTRACT Hydrous partial melting experiments performed between 650 and 750 °C at 200 MPa (H20) on synthetic metapelite compositions (quartz + albite + muscovite + biotite min- eral mixtures) doped with Ba, Sr, Rb, and Cs yielded alkali feldspar crystals with a wide range of compositions in equilibrium at their rims with peraluminous melt. Measured partition coefficients for normally trace lithophile elements between feldspar and melt [D(M)FSP/gl,M = Ba, Sr, Rb, Cs] do not depend on either temperature or bulk composition of melt for the compositions studied. Values of D(Sr)FSP/glare between 10 and 14 and appear to be independent of the albite and orthoclase contents of the feldspar crystals. In contrast, values of D(Ba)FSP/gland D(Rb)FsP/glare strongly dependent on the orthoclase content of feldspar, relationships that can be expressed by the following linear equations: D(Ba)FSP/gl= 0.07 + 0.25(orthoclase) and D(Rb)FsP/gl= 0.03 + 0.0 1(orthoclase), where orthoclase is in mole percent. These equations reproduce the range of previously reported values for D(Ba) and D(Rb) determined on natural and synthetic samples. A single par- tition coefficient for Cs was also determined at D(CS)Fsp/gl= 0.13. These data can be used in conjunction with recently published partition coefficients for muscovite, biotite, and plagioclase feldspars (Blundy and Wood 1991; Icenhower and London 1995) to model quantitatively the trace element signatures of peraluminous mag- mas during anatexis and crystallization. -

Essential Trace Elements in Human Health: a Physician's View
Margarita G. Skalnaya, Anatoly V. Skalny ESSENTIAL TRACE ELEMENTS IN HUMAN HEALTH: A PHYSICIAN'S VIEW Reviewers: Philippe Collery, M.D., Ph.D. Ivan V. Radysh, M.D., Ph.D., D.Sc. Tomsk Publishing House of Tomsk State University 2018 2 Essential trace elements in human health UDK 612:577.1 LBC 52.57 S66 Skalnaya Margarita G., Skalny Anatoly V. S66 Essential trace elements in human health: a physician's view. – Tomsk : Publishing House of Tomsk State University, 2018. – 224 p. ISBN 978-5-94621-683-8 Disturbances in trace element homeostasis may result in the development of pathologic states and diseases. The most characteristic patterns of a modern human being are deficiency of essential and excess of toxic trace elements. Such a deficiency frequently occurs due to insufficient trace element content in diets or increased requirements of an organism. All these changes of trace element homeostasis form an individual trace element portrait of a person. Consequently, impaired balance of every trace element should be analyzed in the view of other patterns of trace element portrait. Only personalized approach to diagnosis can meet these requirements and result in successful treatment. Effective management and timely diagnosis of trace element deficiency and toxicity may occur only in the case of adequate assessment of trace element status of every individual based on recent data on trace element metabolism. Therefore, the most recent basic data on participation of essential trace elements in physiological processes, metabolism, routes and volumes of entering to the body, relation to various diseases, medical applications with a special focus on iron (Fe), copper (Cu), manganese (Mn), zinc (Zn), selenium (Se), iodine (I), cobalt (Co), chromium, and molybdenum (Mo) are reviewed. -
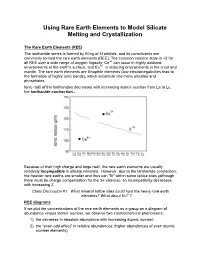
Using Rare Earth Elements to Model Silicate Melting and Crystallization
Using Rare Earth Elements to Model Silicate Melting and Crystallization The Rare Earth Elements (REE) The lanthanide series is formed by filling of 4f orbitals, and its constituents are commonly termed the rare earth elements (REE). The common valence state is +3 for all REE over a wide range of oxygen fugacity; Ce+4 can occur in highly oxidized environments at the earth's surface, and Eu+2 in reducing environments in the crust and mantle. The rare earth elements are lithophile elements (low electronegativities lead to the formation of highly ionic bonds), which substitute into many silicates and phosphates. Ionic radii of the lanthanides decreases with increasing atomic number from La to Lu, the lanthanide contraction… Because of their high charge and large radii, the rare earth elements are usually relatively incompatible in silicate minerals. However, due to the lanthanide contraction, the heavier rare earths are smaller and thus can "fit" within some lattice sites (although there must be charge compensation for the 3+ valence), so incompatibility decreases with increasing Z. Class Discussion #1: What mineral lattice sites could host the heavy rare earth elements? What about Eu2+? REE diagrams If we plot the concentrations of the rare earth elements as a group on a diagram of abundance versus atomic number, we observe two cosmochemical phenomena: 1) the decrease in absolute abundance with increasing atomic number; 2) the “even-odd effect” in relative abundances (higher abundances of even atomic number elements). Because these cosmochemical effects are common to all terrestrial rocks, we would rather ignore them in order to accentuate more subtle variations in absolute and relative concentrations of REE between samples. -
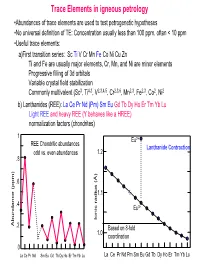
Trace Elements in Igneous Petrology
Trace Elements in igneous petrology •Abundances of trace elements are used to test petrogenetic hypotheses •No universal definition of TE: Concentration usually less than 100 ppm, often < 10 ppm •Useful trace elements: a)First transition series: Sc Ti V Cr Mn Fe Co Ni Cu Zn Ti and Fe are usually major elements, Cr, Mn, and Ni are minor elements Progressive filling of 3d orbitals Variable crystal field stabilization Commonly multivalent (Sc3, Ti4,3, V2,3,4,5, Cr2,3,6, Mn2,3, Fe2,3, Co2, Ni2 b) Lanthanides (REE): La Ce Pr Nd (Pm) Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Light REE and heavy REE (Y behaves like a HREE) normalization factors (chondrites) 1 Eu2+ REE Chondritic abundances Lanthanide Contraction odd vs. even abundances 1.2 .8 .6 1.1 .4 Eu3+ Ionic radius (Å) Ionic radius Abundance (ppm) .2 Based on 8-fold 1.0 coordination 0 La Ce Pr Nd Sm Eu Gd Tb Dy Ho Er Tm Yb Lu La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu (c) Large Ion Lithophile Elements (LILE): may also be partitioned into fluid phase Alkalis: K Rb Cs (monovalent) Alkaline earths: Ba Sr (divalent) Actinides: U, Th, Ra, Pa (multiple valency) (d) High field strength elements (HFSE): small, highly-charged ions Zr, Hf (4 valent) Nb, Ta (4 and 5 valent) (e) Chalcophile elements: Cu, Zn, Pb, Ag, Hg, PGE, (Fe, Co, Ni) (f) Siderophile elements: Fe, Ni, Co, Ge, P, Ga, Au (PGE)… • Decoupled from major elements: lack of stoichiometric constraints (not strictly true) • Goldschmidt’s Rules • Generalities: Incompatible elements are elements that tend to be excluded from common minerals (olivines, pyroxenes, garnets, feldspars, oxides…) in equilibrium with a melt, i.e., they have low D values. -

Implications of Subduction Rehydration for Earth's Deep Water
Implications of Subduction Rehydration for Earth’s Deep Water Cycle Lars Rüpke Physics of Geological Processes, University of Oslo, Oslo, Norway, and SFB 574 Volatiles and Fluids in Subduction Zones, Kiel, Germany Jason Phipps Morgan Cornell University, Ithaca, New York, USA and SFB 574 Volatiles and Fluids in Subduction Zones, Kiel, Germany Jacqueline Eaby Dixon RSMAS/MGG, University of Miami, Miami, Florida, USA The “standard model” for the genesis of the oceans is that they are exhalations from Earth’s deep interior continually rinsed through surface rocks by the global hydrologic cycle. No general consensus exists, however, on the water distribution within the deeper mantle of the Earth. Recently Dixon et al. [2002] estimated water concentrations for some of the major mantle components and concluded that the most primitive (FOZO) are significantly wetter than the recycling associated EM or HIMU mantle components and the even drier depleted mantle source that melts to form MORB. These findings are in striking agreement with the results of numerical modeling of the global water cycle that are presented here. We find that the Dixon et al. [2002] results are consistent with a global water cycle model in which the oceans have formed by efficient outgassing of the mantle. Present-day depleted mantle will contain a small volume fraction of more primitive wet mantle in addition to drier recycling related enriched components. This scenario is consis- tent with the observation that hotspots with a FOZO-component in their source will make wetter basalts than hotspots whose mantle sources contain a larger fraction of EM and HIMU components. -

The Transition-Zone Water Filter Model for Global Material Circulation: Where Do We Stand?
The Transition-Zone Water Filter Model for Global Material Circulation: Where Do We Stand? Shun-ichiro Karato, David Bercovici, Garrett Leahy, Guillaume Richard and Zhicheng Jing Yale University, Department of Geology and Geophysics, New Haven, CT 06520 Materials circulation in Earth’s mantle will be modified if partial melting occurs in the transition zone. Melting in the transition zone is plausible if a significant amount of incompatible components is present in Earth’s mantle. We review the experimental data on melting and melt density and conclude that melting is likely under a broad range of conditions, although conditions for dense melt are more limited. Current geochemical models of Earth suggest the presence of relatively dense incompatible components such as K2O and we conclude that a dense melt is likely formed when the fraction of water is small. Models have been developed to understand the structure of a melt layer and the circulation of melt and vola- tiles. The model suggests a relatively thin melt-rich layer that can be entrained by downwelling current to maintain “steady-state” structure. If deep mantle melting occurs with a small melt fraction, highly incompatible elements including hydro- gen, helium and argon are sequestered without much effect on more compatible elements. This provides a natural explanation for many paradoxes including (i) the apparent discrepancy between whole mantle convection suggested from geophysical observations and the presence of long-lived large reservoirs suggested by geochemical observations, (ii) the helium/heat flow paradox and (iii) the argon paradox. Geophysical observations are reviewed including electrical conductivity and anomalies in seismic wave velocities to test the model and some future direc- tions to refine the model are discussed. -

Evidence for a Carbonatite-Influenced Source Assemblage for Intraplate
minerals Article Evidence for a Carbonatite-Influenced Source Assemblage for Intraplate Basalts from the Buckland Volcanic Province, Queensland, Australia Joshua J. Shea * and Stephen F. Foley Department Earth and Planetary Sciences and ARC Centre of Excellence for Core to Crust Fluid Systems, Macquarie University, North Ryde 2109, New South Wales, Australia * Correspondence: [email protected] Received: 20 June 2019; Accepted: 7 September 2019; Published: 10 September 2019 Abstract: Eastern Australia contains a widespread suite of primitive (MgO 7.5 wt.%) intraplate ≥ basaltic provinces, including those sited along the longest continental hotspot track on Earth ( 2000 km), the Cosgrove track. The Buckland volcanic province is the most southerly basaltic ≈ province on the Cosgrove track before a >1600 km stretch that contains only sparse leucitite volcanism. Buckland is also situated just northeast of the edge of thick cratonic lithosphere where it transitions to a thinner continental lithosphere (<110 km) to the east, which may influence the production of plume-derived melts. Here, analysis of minor and trace elements in olivines in alkali basalts and basanites from the Buckland Province are combined with whole-rock compositions to elucidate the mantle source assemblages, and to calibrate minor and trace element indicators in olivine for application to source mineralogy. Olivine xenocrysts show element concentration ranges typical for peridotites; Mn and Al concentrations indicate that the ambient mantle is spinel, rather than garnet, peridotite. High modal pyroxene content is indicated by high Ni, Zn/Fe, and Fe/Mn in olivines, while high Ti/Sc is consistent with amphibole in the source. Residual phlogopite in the source of the basanites is indicated by low K/Nb in whole rocks, while apatite contains high P2O5 and low Rb/Sr ( 0.015) and Sr/La ( 13). -

Incompatible Element Ratios in Plate Tectonic and Stagnant Lid Planets Kent C. Condie1 and Charles K. Shearer 2. 1Department Of
47th Lunar! and Planetary Science Conference (2016) 1058.pdf Incompatible Element Ratios in Plate Tectonic and Stagnant Lid Planets Kent C. Condie1 and Charles K. Shearer2. 1Department of Earth and Environmental Science, New Mexico Tech, Socorro, New Mexico 87801, 2 Institute of Meteoritics, Department of Earth and Planetary Science, University of New Mexico, Albuquerque, New Mexico 87131, 2 NASA Johnson Space Center, Houston, TX 77058. Introduction: Because ratios of elements with group centered near Earth’s primitive mantle (PM) similar incompatibilities do not change much with composition (Fig. 2). This distribution is similar to degree of melting or fractional crystallization in the the distribution of most mare basalts from the Moon mantles of silicate planets, they are useful in sampled by the Apollo missions, except for the high- characterizing compositions of mantle sources of Ti basalts from Apollo 11 and 17 both of which are basalts that have not interacted with continental crust enriched in Nb. The primitive mantle grouping is [1,2]. Using Nb/Th and Zr/Nb ratios in young especially evident for Apollo 11, 12 and 15 basalts in oceanic basalts, three mantle domains can be which samples cluster tightly around primitive identified [3]: enriched (EM), depleted (DM), and mantle (PM) composition on Zr/Nb-Nb/Th, Th/Yb- hydrated mantle (HM) (Fig. 1). DM is characterized Nb/Yb and La/Sm-Nb/Th diagrams (Fig. 3). Very by high values of both ratios (Nb/Th > 8; Zr/Nb > 20) low-Ti green volcanic glasses (and some due to Th depletion relative to Nb, and to Nb intermediate-Ti yellow volcanic glasses) plot in a depletion relative to Zr. -

Durham Research Online
Durham Research Online Deposited in DRO: 14 March 2018 Version of attached le: Accepted Version Peer-review status of attached le: Peer-reviewed Citation for published item: Namur, Olivier and Humphreys, Madeleine C.S. (2018) 'Trace element constraints on the dierentiation and crystal mush solidication in the Skaergaard intrusion, Greenland.', Journal of petrology., 59 (3). pp. 387-418. Further information on publisher's website: https://doi.org/10.1093/petrology/egy032 Publisher's copyright statement: This is a pre-copyedited, author-produced version of an article accepted for publication in Journal Of Petrology following peer review. The version of record Namur, Olivier Humphreys, Madeleine C.S. (2018). Trace element constraints on the dierentiation and crystal mush solidication in the Skaergaard intrusion, Greenland. Journal of Petrology is available online at: https://doi.org/10.1093/petrology/egy032. Additional information: Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in DRO • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full DRO policy for further details. Durham University Library, Stockton Road, Durham DH1 3LY, United Kingdom Tel : +44 (0)191 334 3042 | Fax : +44 (0)191 334 2971 https://dro.dur.ac.uk Trace element constraints on the differentiation and crystal mush solidification in the Skaergaard intrusion, Greenland Olivier Namur1*, Madeleine C.S. -

NUTRIENTS in DRINKING WATER Manuel Olivares, MD Institute of Nutrition and Food Technology University of Chile Santiago, Chile
4. ESSENTIAL NUTRIENTS IN DRINKING WATER Manuel Olivares, MD Institute of Nutrition and Food Technology University of Chile Santiago, Chile Ricardo Uauy, MD, PhD Institute of Nutrition and Food Technology University of Chile Santiago, Chile London School of Hygiene and Tropical Medicine University of London London, United Kingdom ______________________________________________________________________________________________ I. INTRODUCTION Most of the inorganic chemicals in drinking water are naturally occurring. They are acquired by the contact of water with rocks and soil and the effects of the geological setting, including climate (1-4). However, the chemical composition of drinking water also depends on the contaminating effects of industry, human settlements, agricultural activities and water treatment and distribution (1-4). Depending on water quality at the source, filtration, coagulation, and addition of chemicals to adjust pH and/or control corrosion treatments are employed (1-5). In addition, chlorination or iodination may be used for disinfection and fluoridation for the prevention of dental caries (6-8). Leaching of minerals from metal components used in water treatment plants and plumbing materials occurs when pH and hardness of water are not adjusted. Some of the main sources of dissolved metals include: for Cu- copper or brass plumbing system; Fe- cast iron, steel, and galvanised plumbing system; Zn- zinc galvanised pipes; Ni- chromium- nickel stainless plumbing system; Pb- derived from tin-lead or lead solder; and for Cd- as an impurity in zinc galvanised pipes or cadmium containing solders (1-4,9). Recently, fortification of drinking water has been used in the prevention of iron deficiency in children (10) and to provide iodine in select populations (11).