Mycology in the Canadian Arctic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PALEOLIMNOLOGICAL SURVEY of COMBUSTION PARTICLES from LAKES and PONDS in the EASTERN ARCTIC, NUNAVUT, CANADA an Exploratory Clas
A PALEOLIMNOLOGICAL SURVEY OF COMBUSTION PARTICLES FROM LAKES AND PONDS IN THE EASTERN ARCTIC, NUNAVUT, CANADA An Exploratory Classification, Inventory and Interpretation at Selected Sites NANCY COLLEEN DOUBLEDAY A thesis submitted to the Department of Biology in conformity with the requirements for the degree of Doctor of Philosophy Queen's University Kingston, Ontario, Canada December 1999 Copyright@ Nancy C. Doubleday, 1999 National Library Bibliothèque nationale 1*1 of Canada du Canada Acquisitions and Acquisitions et Bibf iographic Services services bibliographiques 395 Wellington Street 395. rue Wellington Ottawa ON KIA ON4 Ottawa ON K1A ON4 Canada Canada Your lYe Vorre réfhœ Our file Notre refdretua The author has granted a non- L'auteur a accordé une licence non exclusive licence allowing the exclusive pemettant à la National Library of Canada to Bibliothèque nationale du Canada de reproduce, Ioan, distribute or sell reproduire, prêter, distribuer ou copies of this thesis in microform, vendre des copies de cette thèse sous paper or electronic formats. la forme de microfiche/nlm, de reproduction sur papier ou sur format électronique. The author retains ownership of the L'auteur conserve la propriété du copyright in this thesis. Neither the droit d'auteur qui protège cette thèse. thesis nor substantial extracts fiom it Ni la thèse ni des extraits substantiels may be printed or othemise de celle-ci ne doivent être imprimés reproduced without the author's ou autrement reproduits sans son pemission. autorisation. ABSTRACT Recently international attention has been directed to investigation of anthropogenic contaminants in various biotic and abiotic components of arctic ecosystems. Combustion of coai, biomass (charcoal), petroleum and waste play an important role in industrial emissions, and are associated with most hurnan activities. -

Antoniades.2005A.Pdf
Journal of Paleolimnology (2005) 33: 349–360 Ó Springer 2005 -1 Quantitative estimates of recent environmental changes in the Canadian High Arctic inferred from diatoms in lake and pond sediments Dermot Antoniades1,*, Marianne S.V. Douglas1 and John P. Smol2 1Department of Geology, University of Toronto, 22 Russell St., Toronto ON, M5S 3B1, Canada 2Paleoecological Environmental Assessment and Research Lab (PEARL), Department of Biology, Queen’s University, Kingston ON, K7L 3N6, Canada; *Author for correspondence (e-mail: dermot.antoniades@ utoronto.ca) Received 26 May 2004; accepted in revised form 4 November 2004 Key words: Arctic, Climate, Diatoms, Ellef Ringnes Island, Ellesmere Island, Environmental change, Limnology, pH, Proxies Abstract Diatoms were examined in three lacustrine sediment records from Alert, northern Ellesmere Island, and from Isachsen, Ellef Ringnes Island. Diatom assemblages changed markedly since the mid-19th century following relatively stable community composition that spanned centuries to millennia. Three different assemblages, primarily composed of Fragilaria pinnata, Diadesmis spp., or Pinnularia spp., dominated the pre-1850 period at the three sites, but were replaced with different, more diverse assemblages in recent sediments. These species shifts occurred in the mid- to late-19th century in the Isachsen sites, and in the mid- to late-20th century in our Alert site. This difference in timing appears to be a result of the different sensitivities of lakes and ponds to environmental change, rather than of site-specific chemical properties. Reconstructions of pH using diatom inference models indicated increases from 0.5 to 0.8 pH units at these sites over this period of assemblage change. The diatom-inferred pH record from Alert showed agreement with measured climate data from Alert over the last 30 years. -

Arctic Surveillance Civilian Commercial Aerial Surveillance Options for the Arctic
Arctic Surveillance Civilian Commercial Aerial Surveillance Options for the Arctic Dan Brookes DRDC Ottawa Derek F. Scott VP Airborne Maritime Surveillance Division Provincial Aerospace Ltd (PAL) Pip Rudkin UAV Operations Manager PAL Airborne Maritime Surveillance Division Provincial Aerospace Ltd Defence R&D Canada – Ottawa Technical Report DRDC Ottawa TR 2013-142 November 2013 Arctic Surveillance Civilian Commercial Aerial Surveillance Options for the Arctic Dan Brookes DRDC Ottawa Derek F. Scott VP Airborne Maritime Surveillance Division Provincial Aerospace Ltd (PAL) Pip Rudkin UAV Operations Manager PAL Airborne Maritime Surveillance Division Provincial Aerospace Ltd Defence R&D Canada – Ottawa Technical Report DRDC Ottawa TR 2013-142 November 2013 Principal Author Original signed by Dan Brookes Dan Brookes Defence Scienist Approved by Original signed by Caroline Wilcox Caroline Wilcox Head, Space and ISR Applications Section Approved for release by Original signed by Chris McMillan Chris McMillan Chair, Document Review Panel This work was originally sponsored by ARP project 11HI01-Options for Northern Surveillance, and completed under the Northern Watch TDP project 15EJ01 © Her Majesty the Queen in Right of Canada, as represented by the Minister of National Defence, 2013 © Sa Majesté la Reine (en droit du Canada), telle que représentée par le ministre de la Défense nationale, 2013 Preface This report grew out of a study that was originally commissioned by DRDC with Provincial Aerospace Ltd (PAL) in early 2007. With the assistance of PAL’s experience and expertise, the aim was to explore the feasibility, logistics and costs of providing surveillance and reconnaissance (SR) capabilities in the Arctic using private commercial sources. -

Polar Continental Shelf Program Science Report 2019: Logistical Support for Leading-Edge Scientific Research in Canada and Its Arctic
Polar Continental Shelf Program SCIENCE REPORT 2019 LOGISTICAL SUPPORT FOR LEADING-EDGE SCIENTIFIC RESEARCH IN CANADA AND ITS ARCTIC Polar Continental Shelf Program SCIENCE REPORT 2019 Logistical support for leading-edge scientific research in Canada and its Arctic Polar Continental Shelf Program Science Report 2019: Logistical support for leading-edge scientific research in Canada and its Arctic Contact information Polar Continental Shelf Program Natural Resources Canada 2464 Sheffield Road Ottawa ON K1B 4E5 Canada Tel.: 613-998-8145 Email: [email protected] Website: pcsp.nrcan.gc.ca Cover photographs: (Top) Ready to start fieldwork on Ward Hunt Island in Quttinirpaaq National Park, Nunavut (Bottom) Heading back to camp after a day of sampling in the Qarlikturvik Valley on Bylot Island, Nunavut Photograph contributors (alphabetically) Dan Anthon, Royal Roads University: page 8 (bottom) Lisa Hodgetts, University of Western Ontario: pages 34 (bottom) and 62 Justine E. Benjamin: pages 28 and 29 Scott Lamoureux, Queen’s University: page 17 Joël Bêty, Université du Québec à Rimouski: page 18 (top and bottom) Janice Lang, DRDC/DND: pages 40 and 41 (top and bottom) Maya Bhatia, University of Alberta: pages 14, 49 and 60 Jason Lau, University of Western Ontario: page 34 (top) Canadian Forces Combat Camera, Department of National Defence: page 13 Cyrielle Laurent, Yukon Research Centre: page 48 Hsin Cynthia Chiang, McGill University: pages 2, 8 (background), 9 (top Tanya Lemieux, Natural Resources Canada: page 9 (bottom -

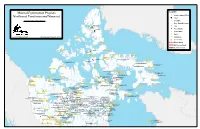
Mineral Exploration Projects Northwest Territories and Nunavut
_ Alert Legend Mineral Exploration Projects &% Nickel-copper PGE's Coal Northwest Territories and Nunavut *# Uranium 0 50 100 200 300 400 500 ` Kilometers Rare Earth Elements 1$ Iron /" Base Metals i[ Active Mine Canada Coal Inc. Fosheim Peninsula ?! Gold _ Eureka XY Diamonds Canada Coal Inc. _ Community Vesle Fiord Winter Road www.miningnorth.com Map Version: May 23, 2012 All Season Road NU-NWT Border _ Isachsen _ Grise Fiord _ Mould Bay _ Dundas Harbour ColtStar Ventures Inc. Eleanor /" _ Polaris Pond Inlet Resolute _ _ _ Clyde River _ Nanisivik _ Commander Resources Ltd. Arctic Bay Storm /" Baffinland Iron Mines Corporation ColtStar Ventures Inc. Mary River _ Qikiqtarjuaq Allen Bay Copper /" 1$ Rio Tinto Canada Exploration Inc. Banks Island Commander Resources Ltd. XY Bravo Lake (Baffin Island Gold) ?! Peregrine Diamonds Ltd. ?! Cumberland Commander Resources Ltd. _ Johnson Point Qimmiq (Baffin Island Gold) XY Fort Ross _ _ Pangnirtung _ Sachs Harbour _ Igloolik Stornoway Diamond Corporation Aviat XY _ Hall Beach Peregrine Diamonds Ltd. Advanced Exploration Inc. 1$ Chidliak Tuktu XY Advanced Exploration Inc. Peregrine Diamonds Ltd. Ulukhaktok 1$ Roche Bay Qilaq _ Advanced Exploration Inc. Tuktoyaktuk Diamonds North Resources Ltd. Western Permits _ _ Cape Parry Halkett Inlet Gold XY _ /" Taloyoak ?! West Melville Iron Company Ltd. Fraser Bay Deposit Vale Canada Limited 1$ Melville Permits /" _ Iqaluit Kugaaruk Darnley Bay Resources Ltd. _ Darnley Bay Diamonds North Resources Ltd. _ Aklavik Diamonds North Resources Ltd. Barrow _ Inuvik _ &% Amaruk XY Paulatuk MMG Resources Inc. &%XY Diamonds North Resources Ltd. Amaruk Nickel ?! Amaruk Gold _ _ Cambridge Bay Gjoa Haven _ Kimmirut _ Fort McPherson Stornoway Diamond Corporation ?! Qilalugaq _ Tsiigehtchic Talmora Diamond Inc. -

Isachsen Area Ellef Ringnes Island District of Franklin Northwest Territories
56-8 CANADA DEPARTMENT OF MINES AND TECHNICAL SURVEYS GEOLOGICAL SURVEY OF CAN ADA . I PAPER 56-8 ISACHSEN AREA ELLEF RINGNES ISLAND DISTRICT OF FRANKLIN NORTHWEST TERRITORIES / ' (Report and Map 15-1956) By W. W. Heywood OTTAWA 19~7 Price, liO cents CANADA DEPARTMENT OF MINES AND TECHNICAL SURVEYS GEOLOGICAL SURVEY OF CANADA Paper 56 - 8 ISACHSEN AREA ELLEF RINGNES ISLAND DISTRICT OF FRANKLIN NORTHWEST TERRITORIES (Report and Map 15 - 1956) By W. W. Heywood OTTAWA 1957 Price, 50 cents CONTENTS Page Introduction •..•.•.••.•.•.•.•• •• .•••••••.••••.••.•••.. Location ...................................... .. Access, travel conditions and present work .••••...• 1 Exploration, history, and previous work ..••••. , •••. 1 Climate ••••••.•••.•••.•••.•••.••••••••.•••••••.. 2 Flora and fauna .• , ...••••••• , , , .•••••.••.••••. , •• 4 Topography and drainage • , • , .•.••••••.•••••.••••.. 4 Acknowledgments ••.••••.•• , , ••.••.••••••••.••••• 5 General geology .••••••• , •••.••••.•.•••.•••••••.••••• 6 Table of formations •• , •••• , •.••••••••.• • •••••••••• 7 Palaeozoic .••.••••.• , •••• , , •.••••• , , .•••••.•.••• 8 Permian or earlier •••.•••••••••.•..••.••.•••. 8 Permian or Carboniferous •••.•• , ••••.••• , ••• , • 8 Lower Cretaceous ..•••••••••.•.••.•••.••••.•••..• 9 Deer Bay formation •.••••.••••• , • , ••.••••• , ••• 9 Isachsen formation .•••••••.••••• , •.••.••••.•• 11 Christopher formation •••••••••••.••.••••••••. 12 Lower or Upper Cretaceous ••.••••.•••• , .••••••••.• 13 Hassel formation •.•••••••••••. , . , •••••••• , •.• 13 Diabase, -

Ski Expedition to the Magnetic North Pole
SKI EXPEDITION TO THE MAGNETIC NORTH POLE One of the most remote and complex expeditions that can be done on Earth A ski crossing departing from the coast of the archipelago of Canada to one of the most remote and inaccessible areas of the planet: the Magnetic North Pole. Located in the middle of the Arctic Ocean, the place is where all compasses point. Currently is located north of the Sverdrup Islands in the area most uninhabited of the whole Canadian Arctic. The Magnetic North Pole, the point that all compasses point, was discovered by John Ross in 1831 in King William Island. Unlike its namesake geographic, the Magnetic Pole moves about 15 kilometers annually. Currently located at latitude 82º North in a very chaotic area of the Arctic Ocean. A plane will take off from Resolute Bay at 75 degrees north latitude and it will drop off the team in the ice pack around 100 kilometersof the Magnetic North Pole. After 11 days of skiing, in the midst of a large expedition atmosphere, surrounded by a white desert in total isolation, we will arrive at our destination. At that time we will be more than 1,000 kilometers from the nearest inhabited place. TRAVEL PROGRAM Day 1 Flight to Ottawa. Overnight at hotel. Day 2 Flight Ottawa to Resolute Bay. Overnight at hotel. Day 3 Day to prepare all equipment and sleds. Overnight at hotel. Day 4 Charter flight in a plane with skis from Resolute to the northern tip of Ellef Ringnes Island where we will landed on the sea ice. -

Joint Arctic Weather Project 19
JOINT ARCTICWEATHER PROJECT By R. W. Rae* INCE Christmas Eve in 1839, when Lieut. C. J. B. Riddell of the Royal S Artilleryinaugurated a systematic program of meteorological and magnetic observations at old Fort York in Toronto, the Meteorological Division of Canada has grown steadily in importance in providing service to the people of Canada. The science of weather forecasting, although not infallible as yet by any means, has improved considerably since the first stormwarning was issued fromthe Toronto office in 1876. The improvement in the accuracy of daily weather forecasts is due not only to advances in meteorological theory but also to a vast increase in coverage provided by weather observing stations. The most important periods of growth of the Meteorological Division occurredduring the following years: In the 1880’s, when telegraphic reporting stations were opened up across thewestern provinces con- currently with the westward extension of the Canadian Pacific Railway; * Meteorological Division, Department of Transport. 18 JOINT ARCTIC WEATHER PROJECT 19 in the 1920’s, when advances in the technique of radio transmission made it possible to install radio reporting stations along the Mackenzie River and in the Hudson Bay and Strait area; and during the years 1947-50, when radio reporting stations were established on some of the remote islands of the Arctic Archipelago. Formany years the region covered by Canada’s Arctic Islands appeared on weather maps as a large blank area. The need for weather reports from this blind spot was recognized but the difficulty and expense involved in the establishment andmaintenance of communitiesin these inaccessible regions were prohibitive. -

Resolute Bay
Qikiqtani Truth Commission Community Histories 1950–1975 Resolute Bay Qikiqtani Inuit Association Published by Inhabit Media Inc. www.inhabitmedia.com Inhabit Media Inc. (Iqaluit), P.O. Box 11125, Iqaluit, Nunavut, X0A 1H0 (Toronto), 146A Orchard View Blvd., Toronto, Ontario, M4R 1C3 Design and layout copyright © 2013 Inhabit Media Inc. Text copyright © 2013 Qikiqtani Inuit Association Photography copyright © 2013 Library and Archives Canada, Northwest Territories Archives, and Tim Kalusha Originally published in Qikiqtani Truth Commission: Community Histories 1950–1975 by Qikiqtani Inuit Association, April 2014. ISBN 978-1-927095-62-1 All rights reserved. The use of any part of this publication reproduced, transmitted in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, or stored in a retrievable system, without written consent of the publisher, is an infringement of copyright law. We acknowledge the support of the Government of Canada through the Department of Canadian Heritage Canada Book Fund program. We acknowledge the support of the Canada Council for the Arts for our publishing program. Please contact QIA for more information: Qikiqtani Inuit Association PO Box 1340, Iqaluit, Nunavut, X0A 0H0 Telephone: (867) 975-8400 Toll-free: 1-800-667-2742 Fax: (867) 979-3238 Email: [email protected] Errata Despite best efforts on the part of the author, mistakes happen. The following corrections should be noted when using this report: Administration in Qikiqtaaluk was the responsibility of one or more federal departments prior to 1967 when the Government of the Northwest Territories was became responsible for the provision of almost all direct services. The term “the government” should replace all references to NANR, AANDC, GNWT, DIAND. -
Mineral Exploration Projects Northwest Territories and Nunavut
_ Alert Legend Mineral Exploration Projects &% Nickel-copper PGE's Northwest Territories and Nunavut Coal #* 0 50 100 200 300 400 500 Kilometers Uranium ` Rare Earth Elements Canada Coal Inc. Fosheim Peninsula $1 Iron _ Eureka /" Canada Coal Inc. Base Metals Vesle Fiord [ i Active Mine ?! Gold XY www.miningnorth.com Map Version: May 23, 2012 _ Isachsen Diamonds _ Community _ Grise Fiord Winter Road All Season Road NU-NWT Border _ Mould Bay ColtStar Ventures Inc. _ Dundas Harbour Eleanor /" _ Polaris _ _ Pond Inlet Resolute _ Clyde River _ _ Nanisivik Commander Resources Ltd. Arctic Bay /" Storm Baffinland Iron Mines Corporation Mary River _ /" Qikiqtarjuaq ColtStar Ventures Inc. $1 Rio Tinto Canada Exploration Inc. Allen Bay Copper Banks Island XY Commander Resources Ltd. ?! Bravo Lake (Baffin Island Gold) Peregrine Diamonds Ltd. ! Cumberland ? Commander Resources Ltd. _ XY Johnson Point _ Qimmiq (Baffin Island Gold) Fort Ross _ Pangnirtung _ Sachs Harbour _ Igloolik Stornoway Diamond Corporation Aviat XY _ Peregrine Diamonds Ltd. Hall Beach Chidliak Advanced Exploration Inc.$1 Tuktu XY Advanced Exploration Inc. Peregrine Diamonds Ltd. $1 Roche Bay _ Qilaq Ulukhaktok Taloyoak _ Tuktoyaktuk _ Diamonds North Resources Ltd. XY Cape Parry Halkett Inlet Gold Advanced Exploration Inc. _ ?! /" Western Permits West Melville Iron Company Ltd$1. /"Vale Canada Limited Darnley Bay Resources Ltd. Fraser Bay Deposit Melville Permits _ Iqaluit Aklavik Darnley Bay _ Diamonds North Resources Ltd. _ &% Kugaaruk XY _ Inuvik _ Paulatuk Barrow Diamonds North Resources Ltd.&%XY MMG Resources Inc. Amaruk ?! Amaruk Nickel _ Diamonds North Resources Ltd. _ Cambridge Bay Gjoa Haven Amaruk Gold Fort McPherson _ _ Kimmirut Talmora Diamond Inc. -

Climatic Regions of the Canadian Arctic Islands J.B
ARCTIC VOL. 34, NO. 3 (SEPTEMBER 198l), P. 225-240 Climatic Regions of the Canadian Arctic Islands J.B. MAXWELL’ ABSTRACT. As a result of a comprehensive assessment of the climate of the Canadian Arctic Islands and adjacent waters, five climatic regions were identified. The regional boundaries were delineated by an analysis of the influence of the major climatic controls while further regional subdivisions were arrived at through consideration of the fields of the standard observed meteorological elements. Short discussions of the climatic characteristics of each sub-region are given and tables outlining values of selected climatic elements are presented. A brief discussion of climatic change across the entire area is included. Key words: Canadian Arctic Islands, climate, climatic change, meteorology RESUME: Suite & une evaluation dttailke du chatdes iles Arctiques canadiennes et des eaux adjacentes, cinq regions climatiques ont kt6 identifikes. Les bornes rtgionale ont CtC tracks au moyen de I’analyse de I’influence des principaux contrbles climatiques, et les subdivisions regionales ont tte dCterminees par I’etude des champs des elhents mkteorologiques standards ayant et6 observes. De courtes discussions des caracttristiquesclimatiques de chaque sous-reion sont presentees, ainsi que destableaux signalant les valeurs de certains des elements climatiques. Une courte discussion du changement climatique dans toute la region parait aussi. Traduit par Maurice Guibord, Le Centre FranGais, The University of Calgary. INTRODUCTION r The vast area of land and water that makes up the Canadian Arctic has long been popularly regarded. as a region of fairly uniform climatic conditions - a “polar desert” with little diversity and strikingly harsh weather throughout the year. -

Xixth CENTURY
CHAPTER V XIXth CENTURY I Boo. — Loyalty Islands, discovered by Capt. Butler in the “ Walpole”,explored in 1828 by Dumont d'Urville. 1800. — Antipodes Islands, discovered by Capt. Woodhouse in H.M.S. “ Reliance” who called them Penantipodes. 、 • S 1801-03. ~ 1 Matthew Flinders, in H.M.S. “ Investigator,,,made a voyage to Terra Australis and explored some coasts of southern Australia. S 1801-1803. ~ ■ !Capt. Nicolas Bandin and F. Peron, in the “ Géographe”,the “ Naturaliste” , ‘‘ Casuarina ”,explore Tasmania, the Great Australian Bay and the west coast of Australia in their voyage ,of discovery to the Austral Lands. 1802. — Murray : exploration of Australia. 1802. — Palmyra Island,discovered by Capt. Sawle,in the American ship “ Palmyra,,. 1803. _ Turnbull : Pacific Archipelagoes (Tuamotu Islands). 1803. ~*.James Stanier Clarke : “ The progress of maritime discovery, from the earliest period to the close of the xvmth Century, forming an extensive system of Hydrogra phyś charts, 10 vignettes, 4 vol., Straham, London. 1803-06. ~ ■ Voyage round, the World by Admiral Krusenstern and Lisiansky in the “ Neva ” and “ Nadesha Exploration of the Carolines, Marshall, Hawaii, Northern coasts of the Pacific and Strait of Bering. 1803-08. — Lieut. W. F. W. Owen, makes a survey of the East Indies. 1804. ~ • Ocean Island (Gilbert Island), discovered by the “ Ocean,,. 1804-06. — Exploration of the Red Sea by Capt. Court in the “ Panther,,• 1804-06. — Captains Lewis and Clarke explore the Missouri River up to its head together with the river head and course of the Columbia River. 1806. — Auckland Island,discovered by Capt. Abraham Bristow in the “ Ocean ” and named after Lord Auckland.