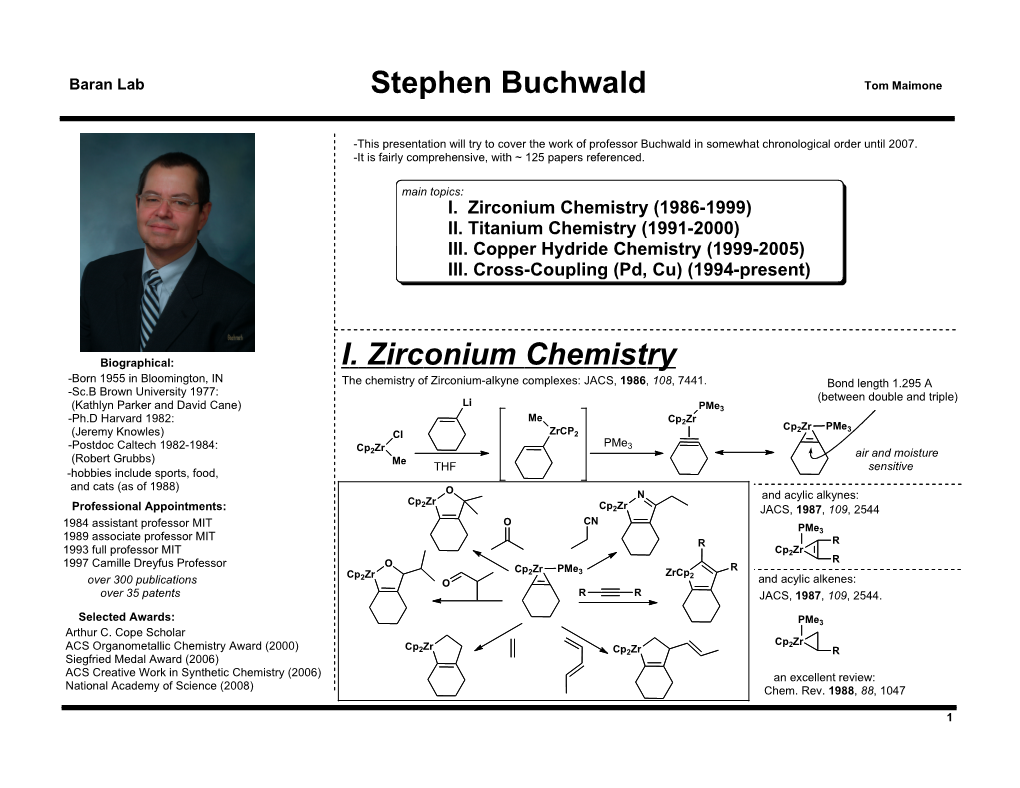
Stephen Buchwald I. Zirconium Chemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Donor/Acceptor Metallocenes: a New Structure Principle in Catalyst Design
COMMUNICATIONS metal complex inside their tunnels, which makes these [19] C. Cascales, E. GutieÂrrez-Puebla, M. A. Monge, C. Ruiz-Valero, materials a good point of departure for designing new Angew. Chem. 1998, 110, 135 ± 138; Angew. Chem. Int. Ed. 1998, 37, 129 ± 131. catalysts; a stable framework after removal of the transition [20] H. Li, M. Eddaaoudi, D. A. Richardson, O. M. Yaghi, J. Am. Chem. metal complex; and large distances between the active metal Soc. 1998, 120, 8567. centers, which allows unhindered access of reactants to these [21] Hailian Li, O. M. Yaghi, J. Am. Chem. Soc. 1998, 120, 10569. centers through uniformly sized 8Rc channels. [22] T. E. Gier, X. Bu, P. Feng, G. D. Stucky, Nature 1998, 395, 154. [23] X. Bu, P. Feng, G. D. Stucky, J. Am. Chem. Soc. 1998, 120, 11204. [24] X. Bu, P. Feng, T. E. Gier, D. Zhao, G. D. Stucky, J. Am. Chem. Soc. 1998, 120, 13389. [25] H. Brumer, K. Wutz, New J. Chem. 1992, 16,57. Experimental Section [26] A. Corma, V. ForneÂs, S. B. Pergher, T. L. Maesennn, J. G. Buglass, Nature 1998, 396, 353. [27] SHELXTL, Siemens Energy & Automation Inc., Analytical Instru- X-ray structure analysis of ICMM-2Cu, ICMM-2Ag, and ICMM-2H: mentation, 1996. Orthorhombic, space group Pnna,MoKa, dimensions of crystals: 0.2 Â 0.1 Â 0.05, 0.02 Â 0.08 Â 0.2, and 0.04 Â 0.16 Â 0.2 mm, respectively; see Table 1 for the cell parameters. Data were collected in a Siemens SMART- CCD diffractometer using w scans over the range 3 < q < 268. -

University of Groningen Structural Characterization of a Cationic Zirconocene Olefin Polymerization Catalyst with Its Methylated
University of Groningen Structural characterization of a cationic zirconocene olefin polymerization catalyst with its methylated boralumoxane counterion Richter, Bodo; Meetsma, Auke; Hessen, Bart; Teuben, Jan H. Published in: Angewandte Chemie-International Edition in English IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2002 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Richter, B., Meetsma, A., Hessen, B., & Teuben, J. H. (2002). Structural characterization of a cationic zirconocene olefin polymerization catalyst with its methylated boralumoxane counterion. Angewandte Chemie-International Edition in English, 41(12), 2166 - 2169. Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum. Download date: 10-02-2018 COMMUNICATIONS A. P. Wheeler, A. Veis, A. I. Caplan, Science 1992, 255, 1098 ± Structural Characterization of a Cationic 1105. [4] P. Calvert, P. -

Organometallic and Catalysis
ORGANOMETALLIC AND CATALYSIS Dr. Malay Dolai, Assistant Professor, Department of Chemistry, Prabhat Kumar College, Contai, Purba Medinipur-721404, WB, India. 1.Introduction Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkaline, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and tin, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide (metal carbonyls), cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. In 1827, Zeise's salt is the first platinum- olefin complex: K[PtCl3(C2H4)].H2O, the first invented organometallic compound. Organometallic compounds find wide use in commercial reactions, both as homogeneous catalysis and as stoichiometric reagents For instance, organolithium, organomagnesium, and organoaluminium compounds, examples of which are highly basic and highly reducing, are useful stoichiometrically, but also catalyze many polymerization reactions. Almost all processes involving carbon monoxide rely on catalysts, notable examples being described as carbonylations. The production of acetic acid from methanol and carbon monoxide is catalyzed via metal carbonyl complexes in the Monsanto process and Cativa process. Most synthetic aldehydes are produced via hydroformylation. The bulk of the synthetic alcohols, at least those larger than ethanol, are produced by hydrogenation of hydroformylation- derived aldehydes. -

Nbcl5-Mg Reagent System in Regio- and Stereoselective Synthesis of (2Z)-Alkenylamines and (3Z)-Alkenylols from Substituted 2-Alkynylamines and 3-Alkynylols
molecules Article NbCl5-Mg Reagent System in Regio- and Stereoselective Synthesis of (2Z)-Alkenylamines and (3Z)-Alkenylols from Substituted 2-Alkynylamines and 3-Alkynylols Rita N. Kadikova *, Azat M. Gabdullin, Oleg S. Mozgovoj, Ilfir R. Ramazanov and Usein M. Dzhemilev Institute of Petrochemistry and Catalysis of Russian Academy of Sciences, 141 Prospekt Oktyabrya, 450075 Ufa, Russia; [email protected] (A.M.G.); [email protected] (O.S.M.); ilfi[email protected] (I.R.R.); [email protected] (U.M.D.) * Correspondence: [email protected] Abstract: The reduction of N,N-disubstituted 2-alkynylamines and substituted 3-alkynylols using the NbCl5–Mg reagent system affords the corresponding dideuterated (2Z)-alkenylamine and (3Z)- alkenylol derivatives in high yields in a regio- and stereoselective manner through the deuterolysis (or hydrolysis). The reaction of substituted propargylamines and homopropargylic alcohols with the in situ generated low-valent niobium complex (based on the reaction of NbCl5 with magnesium metal) is an efficient tool for the synthesis of allylamines and homoallylic alcohols bearing a 1,2-disubstituted double bond. It was found that the well-known approach for the reduction of alkynes based on the use of the TaCl5-Mg reagent system does not work for 2-alkynylamines and 3-alkynylols. Thus, this article reveals a difference in the behavior of two reagent systems—NbCl5-Mg and TaCl5-Mg, Citation: Kadikova, R.N.; Gabdullin, in relation to oxygen- and nitrogen-containing alkynes. A regio- and stereoselective method was A.M.; Mozgovoj, O.S.; Ramazanov, developed for the synthesis of nitrogen-containing E-β-chlorovinyl sulfides based on the reaction of I.R.; Dzhemilev, U.M. -

Room-Temperature Catalytic Hydrodefluorination of Pentafluoro
Journal of Molecular Catalysis A: Chemical 261 (2007) 184–189 Room-temperature catalytic hydrodefluorination of pentafluoro-pyridine by zirconocene fluoro complexes and diisobutylaluminumhydride Ulrike Jager-Fiedler¨ a, Marcus Klahn a, Perdita Arndt a, Wolfgang Baumann a, Anke Spannenberg a, Vladimir V. Burlakov b,1, Uwe Rosenthal a,∗ a Leibniz-Institut f¨ur Katalyse e.V. an der Universit¨at Rostock, Albert-Einstein-Str. 29a, D-18059 Rostock, Germany b A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, Vavilov St. 28, 117813 Moscow, Russia Received 5 May 2006; received in revised form 9 June 2006; accepted 12 June 2006 Available online 11 September 2006 Dedicated to Professor Bernhard Lucke¨ on the occasion of his 70th birthday Abstract 5 Mixtures consisting of zirconocene difluorides Cp2ZrF2 (Cp = substituted or nonsubstituted -cyclopentadienyl) as pre-catalysts and diisobutylaluminumhydride i-Bu2AlH as activator were found to be active catalysts in the room-temperature hydrodefluorination (HDF) of fluorinated pyridines. Evaluation of these systems established rac-(ebthi)ZrF2 (1) and Cp2ZrF2 (3) together with i-Bu2AlH as active catalysts in the room-temperature hydrodefluorination (HDF) of pentafluoro-pyridine. The active species for the conversion were the actually formed hydrides [rac-(ebthi)ZrH(-H)]2 (2) and [Cp2ZrH(-H)]2 (4). The results we obtained (rt, 24 h, turn over number 67) showed a significantly better performance compared to other investigations published before for this HDF reaction. © 2006 Elsevier B.V. All rights reserved. Keywords: Zirconocene; C F bond activation; C H bond activation; Organometallics; Heterocycles 1. Introduction complex Cp2Ti(F)[(O–C(CCF3)3C CF2)] [8]. -

Reactions of Alkyl and Alkenyl Zirconocene Complexes
Reactions of alkyl and alkenyl zirconocene complexes by Klark Thor Hanson A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Chemistry Montana State University © Copyright by Klark Thor Hanson (1992) Abstract: Zirconacycles have been. demonstrated to react sluggishly with acyl chlorides even at elevated temperatures . Studies were undertaken to detertmine whether or not this reaction could be facilitated via transmetallation of one or both of the carbon-zirconium bonds of the zirconacycle to a more suitable metal. This research demonstrates that zirconacycles will react with acyl chlorides in the presence of secondary metallic reagents. Cuprate reagents and catalytic palladium complexes have been demonstrated to function in this capacity, presumably via stoichiometric and catalytic transmetallation respectively. REACTIONS OF ALKYL AND ALKENYL ZIRCONOCENE COMPLEXES by Klark Thor Hanson A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Chemistry MONTANA STATE UNIVERSITY Bozeman, Montana April 1992 ii APPROVAL of a thesis submitted by Klark Thor Hanson This thesis has been read by each member of the thesis committee and has been found to be satisfactory regarding content, English usage, format, citations, bibliographic style, and consistency, and is ready for submission to the College of Graduate Studies. Date Chairperson/ Graduat Committee Approved for the Major Department ^ JC 0- ^ __ Date He$ra.\ Major Department O Approved for the College of Graduate Studies Date I ' Graduate? Dean iii STATEMENT OF PERMISSION TO USE In presenting this thesis in partial fulfillment of the requirements for a master's degree at Montana State University, I agree that the Library shall make it available to borrowers under the rules of the Library. -

Zwitterionic Aluminabenzene-Alkylzirconium Complex Having Half-Zirconocene Structure: Synthesis and Application for an Additive-Free Ethylene Polymerization
ChemComm Zwitterionic Aluminabenzene-Alkylzirconium Complex Having Half-Zirconocene Structure: Synthesis and Application for an Additive-Free Ethylene Polymerization Journal: ChemComm Manuscript ID CC-COM-03-2018-002186 Article Type: Communication Page 1 of 5 Please doChemComm not adjust margins Journal Name COMMUNICATION Zwitterionic Aluminabenzene-Alkylzirconium Complex Having Half-Zirconocene Structure: Synthesis and Application for an Received 00th January 20xx, Additive-Free Ethylene Polymerization Accepted 00th January 20xx a ,b ,b DOI: 10.1039/x0xx00000x Taichi Nakamura, Katsunori Suzuki* and Makoto Yamashita* www.rsc.org/ The aluminabenzene-alkylzirconium complex having half- resulting zwitterionic complexes catalyses ethylene zirconocene structure was synthesized. X-ray crystallographic polymerization as a highly active singlecomponent catalyst.5b analysis of this complex revealed a zwitterionic structure However, the reported group 4 metal catalyst systems with a consisting of cationic alkylzirconium chloride and four-coordinated pendent aluminum-based Lewis acidic moiety were limited,6 aluminate. In the presence of catalytic amount of this complex, while the aluminum additives have been commonly used in ethylene polymerization could proceed without any additives to Kaminsky catalyst. Nomura reported utilization of titanium t form ultra-high molecular weight polyethylene. complex bearing Me2Al(O Bu)(OR) moiety as a single- component catalyst for ethylene polymerization (Scheme In homogeneous catalytic polymerization of olefins, an 1B).6a,b In their plausible mechanism, the dissociation of admixture of group 4 transition-metal complex, such as Me2Al(OtBu)(OR) moiety as an anion generates zwitterionic metallocene or halfmetallocene complexes, and Lewis acidic complex involving cationic titanium centre. This catalyst additive, have been known to construct an effective catalyst system can form high molecular weight PE (> 1,000,000) due 1 system, represented as Kaminsky catalyst. -

Mechanisms of Metal-Mediated Cyclizations
Mechanisms of Metal-Mediated Cyclizations by Benjamin Peter Warner Submitted to the Department of Chemistry in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Organic Chemistry at the Massachusetts Institute of Technology February 1995 © Massachusetts Institute of Technology, 1995 All Rights Reserved Signature of Author.., .... ..,. ....................... ................................................................ Department of Chemistry February 1, 1995 Certified by ........................... I....................... Stephen L. Buchwald Thesis Supervisor Accepted by.............................................v..................................................................... Dietmar Seyferth Chair, Departmental Committee on Graduate Students Sciencx- This doctoral thesis has been examined by a committee of the Department of Chemistry as follows: Professor Gregory C. Fu.............................. .... ........................................................... Chair Professor Stephen L. Buchwald .................. ;................... Thesis Supervisor Professor Christopher C. Cummins.................. ................. .. ...............................; 2 Mechanisms of Metal-Mediated Cyclizations by Benjamin Peter Warner Submitted to the Department of Chemistry in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Organic Chemistry at the Massachusetts Institute of Technology Abstract A complex of zirconocene with two rq2-alkynyl ligands is described. This -

A Rigid Cj-Bridged Ansa-Zirconocene-Derived
A Rigid Cj-Bridged Ansa-Zirconocene-Derived Catalyst System Suited for Stereoselective Low Molecular Weight Polypropylene Formation Gerhard Erker*, Christian Psiorz, Roland Fröhlich Organisch-Chemisches Institut der Universität Münster, Corrensstraße 40, D-48149 Münster Dedicated to Prof. Dr. Dr. h.c. mult. Günther Wilke on the occasion o f his 70th birthday Z. Naturforsch. 50b, 469-475 (1995); received September 20, 1994 Homogeneous Ziegler Catalyst, Isotactic Polypropylene, Ansa-Metallocene, Fulvene 2,5-Hexanedione was converted into the bisfulvene 2, then treated with two molar equiva lents of methyllithium to yield the [4-cyclopentadienylidene-4,7,7-trimethyl-4,5,6,7-tetra- hydroindenyl]dilithio compound 4. Hydrolysis, followed by treatment with acetone/pyrroli dine, gave the corresponding fulvene system 5. Reaction of 5 with methyllithium followed by treatment with ZrCl4 furnished the ring-annulated Cr bridged ansa-metallocene 8, bearing a tert-butyl substituent at the Cp ring, as a 1:1 mixture of two diastereoisomers. Treatment of the fulvene 5 with LiAlH4 followed by ZrCl4 yielded the respective isopropyl-substituted ansa-metallocene diastereomers 9a and 9 b. Com plex 9 b was separated by fractional crys tallization and characterized by X-ray diffraction. Complexes 8 and 9 provide active homo geneous Ziegler-type catalyst systems upon activation with excess methylalumoxane produc ing low molecular weight isotactic polypropylene with high catalyst activities. Introduction Homogeneous group 4 bent metallocene methylalumoxane-derived Ziegler-type catalyst systems have become of enormous importance for the development of a-olefin polymerization [ 1], Most of the catalyst systems of practical impor tance are derived from ansa-metallocene precur I sors [2]. -

Physicochemical Surface-Structure Studies of Highly Active Zirconocene Polymerisation Cite This: Mater
MATERIALS CHEMISTRY FRONTIERS View Article Online RESEARCH ARTICLE View Journal | View Issue Physicochemical surface-structure studies of highly active zirconocene polymerisation Cite this: Mater. Chem. Front., 2020, 4, 3226 catalysts on solid polymethylaluminoxane activating supports† Alexander F. R. Kilpatrick, Nicholas H. Rees, Zoe¨ R. Turner, Jean-Charles Buffet and Dermot O’Hare * Physicochemical surface-structure studies of highly active slurry-phase ethylene polymerisation catalysts has been performed. Zirconocene complexes immobilised on solid polymethylaluminoxane (sMAO) (sMAO–Cp2ZrX2), have been investigated using SEM-EDX, diffuse reflectance FT-IR (DRIFT) and high field (21.1 T) solid state NMR (ssNMR) spectroscopy. The data suggest a common surface-bound cationic 91 Received 14th July 2020, methylzirconocene is the catalytically active species. Zr solid sate NMR spectra of sMAO–Cp2ZrCl2 and Accepted 7th September 2020 Creative Commons Attribution-NonCommercial 3.0 Unported Licence. sMAO–Cp2ZrMe2 are consistent with a common surface-bound Zr environment. However, variation of DOI: 10.1039/d0qm00482k the s-donor (X) groups on the metallocene precatalyst leads to significant differences in polymerisation activity. We report evidence for X group transfer from the precatalyst complex onto the surface of the rsc.li/frontiers-materials aluminoxane support, which in the case of X = C6F5, results in a 38% increase in activity. Introduction benzoic acid.10 We have recently reported the laboratory scale synthesis and detailed characterisation -

Professor Stephen L. Buchwald – This Is Your (Research) Life Literature Review Bobby Brooks Dr Ed Anderson Group 21/06/13 Professor Buchwald – Academic History
Professor Stephen L. Buchwald – This is your (research) life Literature Review Bobby Brooks Dr Ed Anderson Group 21/06/13 Professor Buchwald – Academic history 1955 – Born in Bloomington, Indiana 1977 – Sc.B. Brown University Worked a summer with Prof. Gilbert Stork at Columbia University 1982 – Ph.D. Harvard University: Studying the mechanism of phosphoryl transfer reactions in chemistry and biochemistry under Prof. Jeremy R. Knowles 1982 to 1984 – Myron A. Bantrell postdoctoral fellow at Caltech with Prof. Robert H. Grubbs: Studying titanocene methylenes as reagents in organic synthesis 1984 – Assistant Professor in Chemistry at MIT 1989 – Associate Professor in Chemistry 1993 – Professor in Chemistry 1997 – Camille Dreyfus Professor of Chemistry >350 papers and >50 patents Professor Buchwald and Group – 2000 Awards Award in Organometallic Chemistry from the American Chemical Society Fellow of the American Academy of Arts and Sciences 2005 Bristol-Myers Squibb Distinguished Achievement Award CAS Science Spotlight Award 2006 American Chemical Society's Award for Creative Work in Synthetic Organic Chemistry Siegfried Medal Award in Chemical Methods which Impact Process Chemistry 2008 Elected as a member of the National Academy of Science 2010 Gustavus J. Esselen Award for Chemistry in the Public Interest 2013 Arthur C. Cope Award from the American Chemical Society Harold Edgerton Faculty Achievement Award of MIT Arthur C. Cope Scholar Award MERIT award from the National Institutes of Health Associate editor -

UNIVERSITY of CALIFORNIA Los Angeles Ferrocene-Chelating
UNIVERSITY OF CALIFORNIA Los Angeles Ferrocene-Chelating Heteroscorpionate Ligands Support Zinc Complexes as Redox Switchable Catalysts A thesis submitted in partial satisfaction of the requirements for the degree Master of Science in Chemistry by Tate Christopher Reuter 2018 © Copyright by Tate Christopher Reuter 2018 ABSTRACT OF THE THESIS Ferrocene-Chelating Heteroscorpionate Ligands Support Zinc Complexes as Redox Switchable Catalysts by Tate Christopher Reuter Master of Science in Chemistry University of California, Los Angeles, 2018 Professor Paula Loredana Diaconescu, Chair Ferrocene-chelating heteroscorpionate compounds based on [fc(PPh2)(BH[(3-R-5-R'-1-H)2pz]2)] (fc = 1,1'-ferrocenediyl, pz = pyrazole) are studied and characterized for their role in the synthesis of block copolymers. The ferrocene scaffold is part of a heteroscorpionate ligand that supports late transition metals. A zinc complex, [fc(PPh2)(BH[(3,5-Me-1H)2pz]2)]Zn(μ-OCH2Ph), was synthesized previously and shown to exist in a dimeric state. Herein, the substituents on the pyrazole fragments of the scorpionate are replaced with bulkier groups to force the formation of a monomeric compound in order to arrive at a redox switchable catalyst. ii The thesis of Tate Christopher Reuter is approved. Richard B. Kaner Ellen May Sletten Paula Loredana Diaconescu, Committee Chair University of California, Los Angeles 2018 iii This work is dedicated to my loving family and those making a sacrifice to better themselves and the world we share. iv TABLE OF CONTENTS Abstract ii