Stable Isotope Hydrology
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Rapid Cultural Inventories of Wetlands in Arab States Including Ramsar Sites and World Heritage Properties
Rapid cultural inventories of wetlands in Arab states including Ramsar Sites and World Heritage Properties Building greater understanding of cultural values and practices as a contribution to conservation success Tarek Abulhawa – Lead Author Tricia Cummings – Research and Data Analysis Supported by: May 2017 Acknowledgements The report team expresses their utmost appreciation to Ms. Mariam Ali from the Ramsar Secretariat and Ms. Haifaa Abdulhalim from the Tabe’a Programme (IUCN’s programme in partnership with ARC-WH) for their guidance and support on the preparation of this regional assessment. Special gratitude is extended to all the national focal points from the target countries and sites as well as international experts and colleagues from the Ramsar and IUCN networks for their valuable contributions and reviews of assignment reports drafts. Finally, the team wants to take the opportunity to thank all the peoples of the wetlands in the Arab states for their long established commitment to the protection of their wetlands through their cultural values, traditional knowledge and sustainable practices for the benefit of future generations. Cover: Traditional felucca fishing boat, Tunisia. DGF Tunisa Contents Executive summary . 4 Introduction . 9 Methodology . 13 Assessment Results . 21 Algeria . 23 La Vallée d’Iherir . 24 Oasis de Tamantit et Sid Ahmed Timmi. 27 Réserve Intégrale du Lac Tonga . 32 Egypt . 35 Lake Bardawil . 36 Lake Burullus . 41 Wadi El Rayan Protected Area . 44 Iraq . 49 Central Marshes . 52 Hammar Marshes . 55 Hawizeh Marshes . 58 Mauritania . 63 Lac Gabou et le réseau hydrographique du Plateau du Tagant . 64 Parc National du Banc d’Arguin . 67 Parc National du Diawling . -

Local Topography Increasingly Influences the Mass Balance of A
Local topography increasingly influences the mass balance of a retreating cirque glacier Caitlyn Florentine1, 2, Joel Harper2, Daniel Fagre1, Johnnie Moore2, Erich Peitzsch1 1U.S. Geological Survey, Northern Rocky Mountain Science Center, West Glacier, Montana, 59936, USA 5 2Department of Geosciences, University of Montana, Missoula, Montana, 59801, USA Correspondence to: Caitlyn Florentine ([email protected]) Abstract. Local topographically driven processes, such as wind drifting, avalanching, and shading, are known to alter the relationship between the mass balance of small cirque glaciers and regional climate. Yet partitioning such local effects from regional climate influence has proven difficult, creating uncertainty in the climate representativeness of some glaciers. We 10 address this problem for Sperry Glacier in Glacier National Park, USA using field-measured surface mass balance, geodetic constraints on mass balance, and regional climate data recorded at a network of meteorological and snow stations. Geodetically derived mass changes between 1950-1960, 1960-2005, and 2005-2014 document average mass change rates during each period at -0.22±0.12 m w.e. yr-1, -0.18±0.05 m w.e. yr-1, and -0.10±0.03 m w.e. yr-1. A correlation of field- measured mass balance and regional climate variables closely (i.e. within 0.08 m w.e. yr-1) predicts the geodetically 15 measured mass loss from 2005-2014. However, this correlation overestimates glacier mass balance for 1950-1960 by +1.18±0.92 m w.e. yr-1. Our analysis suggests that local effects, not represented in regional climate variables, have become a more dominant driver of the net mass balance as the glacier lost 0.50 km2 and retreated further into its cirque. -

Pulse-To-Pulse Coherent Doppler Measurements of Waves and Turbulence
1580 JOURNAL OF ATMOSPHERIC AND OCEANIC TECHNOLOGY VOLUME 16 Pulse-to-Pulse Coherent Doppler Measurements of Waves and Turbulence FABRICE VERON AND W. K ENDALL MELVILLE Scripps Institution of Oceanography, University of California, San Diego, La Jolla, California (Manuscript received 7 August 1997, in ®nal form 12 September 1998) ABSTRACT This paper presents laboratory and ®eld testing of a pulse-to-pulse coherent acoustic Doppler pro®ler for the measurement of turbulence in the ocean. In the laboratory, velocities and wavenumber spectra collected from Doppler and digital particle image velocimeter measurements compare very well. Turbulent velocities are obtained by identifying and ®ltering out deep water gravity waves in Fourier space and inverting the result. Spectra of the velocity pro®les then reveal the presence of an inertial subrange in the turbulence generated by unsteady breaking waves. In the ®eld, comparison of the pro®ler velocity records with a single-point current measurement is satisfactory. Again wavenumber spectra are directly measured and exhibit an approximate 25/3 slope. It is concluded that the instrument is capable of directly resolving the wavenumber spectral levels in the inertial subrange under breaking waves, and therefore is capable of measuring dissipation and other turbulence parameters in the upper mixed layer or surface-wave zone. 1. Introduction ence on the measurement technique. For example, pro- ®ling instruments have been very successful in mea- The surface-wave zone or upper surface mixed layer suring microstructure at greater depth (Oakey and Elliot of the ocean has received considerable attention in re- 1982; Gregg et al. 1993), but unless the turnaround time cent years. -
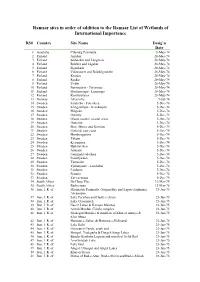
Ramsar Sites in Order of Addition to the Ramsar List of Wetlands of International Importance
Ramsar sites in order of addition to the Ramsar List of Wetlands of International Importance RS# Country Site Name Desig’n Date 1 Australia Cobourg Peninsula 8-May-74 2 Finland Aspskär 28-May-74 3 Finland Söderskär and Långören 28-May-74 4 Finland Björkör and Lågskär 28-May-74 5 Finland Signilskär 28-May-74 6 Finland Valassaaret and Björkögrunden 28-May-74 7 Finland Krunnit 28-May-74 8 Finland Ruskis 28-May-74 9 Finland Viikki 28-May-74 10 Finland Suomujärvi - Patvinsuo 28-May-74 11 Finland Martimoaapa - Lumiaapa 28-May-74 12 Finland Koitilaiskaira 28-May-74 13 Norway Åkersvika 9-Jul-74 14 Sweden Falsterbo - Foteviken 5-Dec-74 15 Sweden Klingavälsån - Krankesjön 5-Dec-74 16 Sweden Helgeån 5-Dec-74 17 Sweden Ottenby 5-Dec-74 18 Sweden Öland, eastern coastal areas 5-Dec-74 19 Sweden Getterön 5-Dec-74 20 Sweden Store Mosse and Kävsjön 5-Dec-74 21 Sweden Gotland, east coast 5-Dec-74 22 Sweden Hornborgasjön 5-Dec-74 23 Sweden Tåkern 5-Dec-74 24 Sweden Kvismaren 5-Dec-74 25 Sweden Hjälstaviken 5-Dec-74 26 Sweden Ånnsjön 5-Dec-74 27 Sweden Gammelstadsviken 5-Dec-74 28 Sweden Persöfjärden 5-Dec-74 29 Sweden Tärnasjön 5-Dec-74 30 Sweden Tjålmejaure - Laisdalen 5-Dec-74 31 Sweden Laidaure 5-Dec-74 32 Sweden Sjaunja 5-Dec-74 33 Sweden Tavvavuoma 5-Dec-74 34 South Africa De Hoop Vlei 12-Mar-75 35 South Africa Barberspan 12-Mar-75 36 Iran, I. R. -

Power Sources Challenge
POWER SOURCES CHALLENGE FUSION PHYSICS! A CLEAN ENERGY Summary: What if we could harness the power of the Sun for energy here on Fusion reactions occur when two nuclei come together to form one Earth? What would it take to accomplish this feat? Is it possible? atom. The reaction that happens in the sun fuses two Hydrogen atoms together to produce Helium. It looks like this in a very simplified way: Many researchers including our Department of Energy scientists and H + H He + ENERGY. This energy can be calculated by the famous engineers are taking on this challenge! In fact, there is one DOE Laboratory Einstein equation, E = mc2. devoted to fusion physics and is committed to being at the forefront of the science of magnetic fusion energy. Each of the colliding hydrogen atoms is a different isotope of In order to understand a little more about fusion energy, you will learn about hydrogen, one deuterium and one the atom and how reactions at the atomic level produce energy. tritium. The difference in these isotopes is simply one neutron. Background: It all starts with plasma! If you need to learn more about plasma Deuterium has one proton and one physics, visit the Power Sources Challenge plasma activities. neutron, tritium has one proton and two neutrons. Look at the The Fusion Reaction that happens in the SUN looks like this: illustration—do you see how the mass of the products is less than the mass of the reactants? That is called a mass deficit and that difference in mass is converted into energy. -

Characteristics of the Bergschrund of an Avalanche-Cone Glacier in the Canadian Rocky Mountains
JOlIl"lla/ o/G/aci%gl'. VoL 29. No. 10 1. 1983 CHARACTERISTICS OF THE BERGSCHRUND OF AN AVALANCHE-CONE GLACIER IN THE CANADIAN ROCKY MOUNTAINS By G ERALD OSBORN (Department of Geology and Geophysics, Uni versity o f Calgary, Calgary, Alberta T2N I N4, Canada) ABSTRACT. Fi eld study of th e bergschrund of a small avalanche-cone glacier at the base of Mt Chephren, in Banff Nati onal Park , has been ca rried out as part of a general ex pl oratory study of glacier-head crevasses in th e Canadi an Roc ki es. The bergsc hrun d consists of a wide. shall ow. partl y bedrock-fl oored gap, und erneath whi ch ex tends a nearl y vertical Ralldklu!I, and a small , offset, subsidi ary crevasse (or crevasses). The fo ll owin g observations rega rdin g the behavior of th e bergsc hruncl and ice adjacent to it are of parti cul ar interest: ( I) topograph y of the subglaeial bedrock is a control on the location of the main bergschrund and subsidi a ry crevasses. (2) th e main bergschrund and subsid ia ry crevasse(s) are conn ected by subglacial gaps betwee n bedrock and ice; th e gaps are part of th e "bergschrund system" , (3) snow/ ice immedi ately down-glacier of the bergschrund system moves nea rl y verticall y dow nwa rd in response to rotational fl ow of the glacier. a ll owin g the bergschrund components to keep the same location and size fro m year to year, (4) an inde pend ent accumul ati on, fl ow. -

Glossary Glossary
Glossary Glossary Albedo A measure of an object’s reflectivity. A pure white reflecting surface has an albedo of 1.0 (100%). A pitch-black, nonreflecting surface has an albedo of 0.0. The Moon is a fairly dark object with a combined albedo of 0.07 (reflecting 7% of the sunlight that falls upon it). The albedo range of the lunar maria is between 0.05 and 0.08. The brighter highlands have an albedo range from 0.09 to 0.15. Anorthosite Rocks rich in the mineral feldspar, making up much of the Moon’s bright highland regions. Aperture The diameter of a telescope’s objective lens or primary mirror. Apogee The point in the Moon’s orbit where it is furthest from the Earth. At apogee, the Moon can reach a maximum distance of 406,700 km from the Earth. Apollo The manned lunar program of the United States. Between July 1969 and December 1972, six Apollo missions landed on the Moon, allowing a total of 12 astronauts to explore its surface. Asteroid A minor planet. A large solid body of rock in orbit around the Sun. Banded crater A crater that displays dusky linear tracts on its inner walls and/or floor. 250 Basalt A dark, fine-grained volcanic rock, low in silicon, with a low viscosity. Basaltic material fills many of the Moon’s major basins, especially on the near side. Glossary Basin A very large circular impact structure (usually comprising multiple concentric rings) that usually displays some degree of flooding with lava. The largest and most conspicuous lava- flooded basins on the Moon are found on the near side, and most are filled to their outer edges with mare basalts. -

Chester County Marriages Bride Index 1885-1930
Chester County Marriages Bride Index 1885-1930 Bride's Last Name Bride's First Name Bride's Middle Bride's Date of Birth Bride's Age Groom's First Groom's Last Date of Application Date of Marriage Place of Marriage License # Dabney Ruth 47 Arthur Garner October 16, 1929 West Chester 29675 Dabundo Louise 18 Saverio DiMaio December 10, 1925 West Chester 26115.5 Dadley Fannie K 23 Albert Smith April 12, 1916 Toughkenamon 19118 Dagastina LorenzaFebruary 6, 1889 Michele Mastragiolo March 16, 1908 Norristown 13663 Dagne Eva EJuly 8, 1874 Jesse Downs December 27, 1899 West Chester 7490 Dagostina Philomena 18 Nicholas Tuscano August 2, 1925 Phoenixville 25847 D'Agostino Angelina 28 Gabriele Natale April 19, 1915 Norristown 18401 Dague Anna LSeptember 23, 1884 James Porter December 18, 1907 Parkesburg 13097 Dague CoraNovember 10, 1874 Vernon Powell February 10, 1904 Lionville 10244 Dague Lillie AApril 27, 1873 Frederick Gottier April 7, 1902 West Chester 9034 Dague M KatieJanuary 1, 1872 Charles Gantt April 17, 1900 Downington 7673 Dague Mary J 29 Ralph Young March 5, 1921 Coatesville 22856 Dague Sara Ellen 36 Rees Helms October 4, 1922 Honey Brook 23933 Dague Sarah Emma1858 James Eppihimer January 14, 1886 West Chester 104 Dahl Olga G 23 Claude Prettyman January 24, 1925 West Chester 25559 Dahms Elsie Annie 26 Chester Kirkhoff October 31, 1929 Pottstown 29710 Dailey Agnes1859 John McCarthy January 13, 1886 West Chester 084 Dailey Anna 19 Rhinehart Merkt August 14, 1913 Downingtown 17216 Dailey Anna RApril 29, 1877 18 Thomas Argne January 4, 1896 -

Fire Island—Historical Background
Chapter 1 Fire Island—Historical Background Brief Overview of Fire Island History Fire Island has been the location for a wide variety of historical events integral to the development of the Long Island region and the nation. Much of Fire Island’s history remains shrouded in mystery and fable, including the precise date at which the barrier beach island was formed and the origin of the name “Fire Island.” What documentation does exist, however, tells an interesting tale of Fire Island’s progression from “Shells to Hotels,” a phrase coined by one author to describe the island’s evo- lution from an Indian hotbed of wampum production to a major summer resort in the twentieth century.1 Throughout its history Fire Island has contributed to some of the nation’s most important historical episodes, including the development of the whaling industry, piracy, the slave trade, and rumrunning. More recently Fire Island, home to the Fire Island National Seashore, exemplifies the late twentieth-century’s interest in preserving natural resources and making them available for public use. The Name. It is generally believed that Fire Island received its name from the inlet that cuts through the barrier and connects the Great South Bay to the ocean. The name Fire Island Inlet is seen on maps dating from the nineteenth century before it was attributed to the barrier island. On September 15, 1789, Henry Smith of Boston sold a piece of property to several Brookhaven residents through a deed that stated the property ran from “the Head of Long Cove to Huntting -

The Dole Effect and Its Variations During the Last 130,000 Years As Measured in the Vostok Ice Core
GLOBAL BIOGEOCHEMICAL CYCLES, VOL. 8, NO. 3, PAGES 363-376, SEPTEMBER 1994 The Dole effect and its variations during the last 130,000 years as measured in the Vostok ice core Michael Bender • and Todd Sowers 2 GraduateSchool of Oceanography,University of RhodeIsland, Kingston Laurent Labeyrie Centredes Faibles Radioactivites, Centre National de la RechercheScientifique, Commissariat a L'EnergieAtomique, Gif-sur- Yvette, France Abstract. We review the currentunderstanding of the Dole effect (the observeddifference between the •5180of atmospheric0 2 andthat of seawater)and its causes, extend the record of variationsin theDole effectback to 130kyr before present using data on the •5180 of 0 2obtained from studying the Vostok ice core(Sowers et al., 1993), anddiscuss the significanceof temporalvariations. The Dole effectreflects oxygenisotope fractionation during photosynthesis, respiration, and hydrologic processes (evaporation, precipitation,and evapotranspiration). Our bestprediction of the present-dayDole effect,+20.8 %0,is considerablylower thanthe observedvalue, + 23.5 %0,and we discusspossible causes of this discrepancy. During the past 130 kyr, the Dole effecthas been 0.05 %0lower thanthe present value, on average.The standarddeviation of the Dole effect from the meanhas been only _+0.2 %0,and the Dole effect is nearly unchangedbetween glacial maxima and interglacial periods. The smallvariability in theDole effect suggeststhat relative rates of primaryproduction in theland and marine realms have been relatively constant.Most periodicvariability in the Dole effectis in the precessionband, suggesting that changes in thisglobal biogeochemical term reflects variations in low-latitudeland hydrology and productivity or possiblyvariability in low-latitudeoceanic productivity. Introduction they can potentially be made to do so. Ice core reconstructionsof the concentrationsof bioactivegases in air Our understanding of the response of the biosphere to provide an integratedglobal signal which complementsthe Pleistoceneclimate change is actually quite limited. -

Proceedings Issn 2654-1823
SAFEGREECE CONFERENCE PROCEEDINGS ISSN 2654-1823 14-17.10 proceedings SafeGreece 2020 – 7th International Conference on Civil Protection & New Technologies 14‐16 October, on‐line | www.safegreece.gr/safegreece2020 | [email protected] Publisher: SafeGreece [www.safegreece.org] Editing, paging: Katerina – Navsika Katsetsiadou Title: SafeGreece 2020 on‐line Proceedings Copyright © 2020 SafeGreece SafeGreece Proceedings ISSN 2654‐1823 SafeGreece 2020 on-line Proceedings | ISSN 2654-1823 index About 1 Committees 2 Topics 5 Thanks to 6 Agenda 7 Extended Abstracts (Oral Presentations) 21 New Challenges for Multi – Hazard Emergency Management in the COVID-19 Era in Greece Evi Georgiadou, Hellenic Institute for Occupational Health and Safety (ELINYAE) 23 An Innovative Emergency Medical Regulation Model in Natural and Manmade Disasters Chih-Long Pan, National Yunlin University of Science and technology, Taiwan 27 Fragility Analysis of Bridges in a Multiple Hazard Environment Sotiria Stefanidou, Aristotle University of Thessaloniki 31 Nature-Based Solutions: an Innovative (Though Not New) Approach to Deal with Immense Societal Challenges Thanos Giannakakis, WWF Hellas 35 Coastal Inundation due to Storm Surges on a Mediterranean Deltaic Area under the Effects of Climate Change Yannis Krestenitis, Aristotle University of Thessaloniki 39 Optimization Model of the Mountainous Forest Areas Opening up in Order to Prevent and Suppress Potential Forest Fires Georgios Tasionas, Democritus University of Thrace 43 We and the lightning Konstantinos Kokolakis, -

The Problem of the Brisbane Tuff
50 VoL. XLV., No. 11. The Problem of the Brisbane Tuff. By PROFESSOR H. C. RICHARDS, D. Sc., and W. H. BRYAN, D. Sc. (Department of Geology, University of Queensland). PLATES IV.-VI. ( Accepted for publication by the Roya� Society of Qiu!ensland, 30th November, 1933). · 1. INTRODUCTION. The following paper recor�s an attempt to discover the ongm, and explain the mode of formation of the rock now known as the Brisbane Tuff. While the authors long ago realised that this rock possessed a number of unusual features, their doubts as to its true nature were revived and strengthened when a section showing well developed columnar structure was exposed in the municipal quarry at Windsor some three miles north of the centre of the city of Brisbane. A more detailed examination disclosed other anomalous features, but a search of the literature showed that the characters displayed by the Brisbane Tuff, although rare, were not unique but could be closely paralleled in the "Great Hot Sand Flow" of Alaska and in the "Ignimbrites " of New Zealand, while they also resembled in certain respects the " Nitees ardentes" of Mt. Pelee. In the pages which follow only those features that have a direct bearing on the origin and nature of the Eris bane Tuff will be con sidered. For a more general account the reader is referred. to the paper by Mrs. C. Briggs (1928) published in these proceedings. 2. EARLIER VIEWS AS TO ORIGIN AND MODE OF FORMATION. The rock which is now knpwn as the Eris bane Tuff was first described by Leichhardt (1855) who examined the rock on his visit to Brisbane in 1844.