Gonococcemia in an Immune-Suppressed Patient Receiving Eculizumab
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

WO 2014/134709 Al 12 September 2014 (12.09.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/134709 Al 12 September 2014 (12.09.2014) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 31/05 (2006.01) A61P 31/02 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/CA20 14/000 174 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 4 March 2014 (04.03.2014) KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (25) Filing Language: English OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (26) Publication Language: English SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (30) Priority Data: ZW. 13/790,91 1 8 March 2013 (08.03.2013) US (84) Designated States (unless otherwise indicated, for every (71) Applicant: LABORATOIRE M2 [CA/CA]; 4005-A, rue kind of regional protection available): ARIPO (BW, GH, de la Garlock, Sherbrooke, Quebec J1L 1W9 (CA). GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, (72) Inventors: LEMIRE, Gaetan; 6505, rue de la fougere, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, Sherbrooke, Quebec JIN 3W3 (CA). -

| Oa Tai Ei Rama Telut Literatur
|OA TAI EI US009750245B2RAMA TELUT LITERATUR (12 ) United States Patent ( 10 ) Patent No. : US 9 ,750 ,245 B2 Lemire et al. ( 45 ) Date of Patent : Sep . 5 , 2017 ( 54 ) TOPICAL USE OF AN ANTIMICROBIAL 2003 /0225003 A1 * 12 / 2003 Ninkov . .. .. 514 / 23 FORMULATION 2009 /0258098 A 10 /2009 Rolling et al. 2009 /0269394 Al 10 /2009 Baker, Jr . et al . 2010 / 0034907 A1 * 2 / 2010 Daigle et al. 424 / 736 (71 ) Applicant : Laboratoire M2, Sherbrooke (CA ) 2010 /0137451 A1 * 6 / 2010 DeMarco et al. .. .. .. 514 / 705 2010 /0272818 Al 10 /2010 Franklin et al . (72 ) Inventors : Gaetan Lemire , Sherbrooke (CA ) ; 2011 / 0206790 AL 8 / 2011 Weiss Ulysse Desranleau Dandurand , 2011 /0223114 AL 9 / 2011 Chakrabortty et al . Sherbrooke (CA ) ; Sylvain Quessy , 2013 /0034618 A1 * 2 / 2013 Swenholt . .. .. 424 /665 Ste - Anne -de - Sorel (CA ) ; Ann Letellier , Massueville (CA ) FOREIGN PATENT DOCUMENTS ( 73 ) Assignee : LABORATOIRE M2, Sherbrooke, AU 2009235913 10 /2009 CA 2567333 12 / 2005 Quebec (CA ) EP 1178736 * 2 / 2004 A23K 1 / 16 WO WO0069277 11 /2000 ( * ) Notice : Subject to any disclaimer, the term of this WO WO 2009132343 10 / 2009 patent is extended or adjusted under 35 WO WO 2010010320 1 / 2010 U . S . C . 154 ( b ) by 37 days . (21 ) Appl. No. : 13 /790 ,911 OTHER PUBLICATIONS Definition of “ Subject ,” Oxford Dictionary - American English , (22 ) Filed : Mar. 8 , 2013 Accessed Dec . 6 , 2013 , pp . 1 - 2 . * Inouye et al , “ Combined Effect of Heat , Essential Oils and Salt on (65 ) Prior Publication Data the Fungicidal Activity against Trichophyton mentagrophytes in US 2014 /0256826 A1 Sep . 11, 2014 Foot Bath ,” Jpn . -

Online Appendix: Previous Methods Used to Generate National Estimates of Incidence and Mortality
Online Appendix: Previous methods used to generate national estimates of incidence and mortality Angus, et al. Crit Care Med 2001; 29:1303-1310. ICD-9 Infection ICD-9 Acute Organ Dysfution 001, Cholera 785.5 Shock without trauma 002, Typhoid/paratyphoid 458 Hypotension fever 96.7 Mechanical ventilation 003, Other salmonella infection; 348.3 Encephalopathy 004, Shigellosis 293Transient organic psychosis 005, Other food poisoning; 348.1 Anoxic brain damage 008, Intestinal infection not otherwise classified; 287.4 Secondary thrombocytopenia 009, Ill-defined intestinal infection 287.5Thrombocytopenia, unspecified 010, Primary tuberculosis infection 286.9 Other/unspecified coagulation defect 011, Pulmonary tuberculosis; 286.6 Defibrination syndrome 012, Other respiratory tuberculosis; 570 Acute and subacute necrosis of liver 013, Central nervous system tuberculosis; 573.4 Hepatic infarction 014, Intestinal tuberculosis; 584 Acute renal failure 015, Tuberculosis of bone and joint; 016, Genitourinary tuberculosis; 017, Tuberculosis not otherwise classified; 018, Miliary tuberculosis; 020, Plague; 021, Tularemia; 022, Anthrax; 023, Brucellosis; 024, Glanders; 025, Melioidosis; 026, Rat-bite fever; 027, Other bacterial zoonoses; 030, Leprosy; 031, Other mycobacterial disease; 032, Diphtheria; 033, Whooping cough; 034, Streptococcal throat/scarlet fever; 035, Erysipelas; 036, Meningococcal infection; 037, Tetanus; 038, Septicemia; 039, Actinomycotic infections; 040, Other bacterial diseases; 041, Bacterial infection in other diseases not otherwise -

INFECTIOUS DISEASES of HAITI Free
INFECTIOUS DISEASES OF HAITI Free. Promotional use only - not for resale. Infectious Diseases of Haiti - 2010 edition Infectious Diseases of Haiti - 2010 edition Copyright © 2010 by GIDEON Informatics, Inc. All rights reserved. Published by GIDEON Informatics, Inc, Los Angeles, California, USA. www.gideononline.com Cover design by GIDEON Informatics, Inc No part of this book may be reproduced or transmitted in any form or by any means without written permission from the publisher. Contact GIDEON Informatics at [email protected]. ISBN-13: 978-1-61755-090-4 ISBN-10: 1-61755-090-6 Visit http://www.gideononline.com/ebooks/ for the up to date list of GIDEON ebooks. DISCLAIMER: Publisher assumes no liability to patients with respect to the actions of physicians, health care facilities and other users, and is not responsible for any injury, death or damage resulting from the use, misuse or interpretation of information obtained through this book. Therapeutic options listed are limited to published studies and reviews. Therapy should not be undertaken without a thorough assessment of the indications, contraindications and side effects of any prospective drug or intervention. Furthermore, the data for the book are largely derived from incidence and prevalence statistics whose accuracy will vary widely for individual diseases and countries. Changes in endemicity, incidence, and drugs of choice may occur. The list of drugs, infectious diseases and even country names will vary with time. © 2010 GIDEON Informatics, Inc. www.gideononline.com All Rights Reserved. Page 2 of 314 Free. Promotional use only - not for resale. Infectious Diseases of Haiti - 2010 edition Introduction: The GIDEON e-book series Infectious Diseases of Haiti is one in a series of GIDEON ebooks which summarize the status of individual infectious diseases, in every country of the world. -

Dermatological Indications of Disease - Part II This Patient on Dialysis Is Showing: A
“Cutaneous Manifestations of Disease” ACOI - Las Vegas FR Darrow, DO, MACOI Burrell College of Osteopathic Medicine This 56 year old man has a history of headaches, jaw claudication and recent onset of blindness in his left eye. Sed rate is 110. He has: A. Ergot poisoning. B. Cholesterol emboli. C. Temporal arteritis. D. Scleroderma. E. Mucormycosis. Varicella associated. GCA complex = Cranial arteritis; Aortic arch syndrome; Fever/wasting syndrome (FUO); Polymyalgia rheumatica. This patient missed his vaccine due at age: A. 45 B. 50 C. 55 D. 60 E. 65 He must see a (an): A. neurologist. B. opthalmologist. C. cardiologist. D. gastroenterologist. E. surgeon. Medscape This 60 y/o male patient would most likely have which of the following as a pathogen? A. Pseudomonas B. Group B streptococcus* C. Listeria D. Pneumococcus E. Staphylococcus epidermidis This skin condition, erysipelas, may rarely lead to septicemia, thrombophlebitis, septic arthritis, osteomyelitis, and endocarditis. Involves the lymphatics with scarring and chronic lymphedema. *more likely pyogenes/beta hemolytic Streptococcus This patient is susceptible to: A. psoriasis. B. rheumatic fever. C. vasculitis. D. Celiac disease E. membranoproliferative glomerulonephritis. Also susceptible to PSGN and scarlet fever and reactive arthritis. Culture if MRSA suspected. This patient has antithyroid antibodies. This is: • A. alopecia areata. • B. psoriasis. • C. tinea. • D. lichen planus. • E. syphilis. Search for Hashimoto’s or Addison’s or other B8, Q2, Q3, DRB1, DR3, DR4, DR8 diseases. This patient who works in the electronics industry presents with paresthesias, abdominal pain, fingernail changes, and the below findings. He may well have poisoning from : A. lead. B. -

Dermatology Grand Rounds 2019 Skin Signs of Internal Disease
Dermatology Grand Rounds 2019 skin signs of internal disease John Strasswimmer, MD, PhD Affiliate Clinical Professor (Dermatology), FAU College of Medicine Research Professor of Biochemistry, FAU College of Science Associate Clinical Professor, U. Miami Miller School of Medicine Dermatologist and Internal Medicine “Normal” abnormal skin findings in internal disease • Thyroid • Renal insufficiency • Diabetes “Abnormal” skin findings as clue to internal disease • Markers of infectious disease • Markers of internal malignancy risk “Consultation Cases” • Very large dermatology finding • A very tiny dermatology finding Dermatologist and Internal Medicine The "Red and Scaly” patient “Big and Small” red rashes not to miss The "Red and Scaly” patient • 29 Year old man with two year pruritic eruption • PMHx: • seasonal allergies • childhood eczema • no medications Erythroderma Erythroderma • Also called “exfoliative dermatitis” • Not stevens-Johnson / toxic epidermal necrosis ( More sudden onset, associated with target lesions, mucosal) • Generalized erythema and scale >80-90% of body surface • May be associated with telogen effluvium It is not a diagnosis per se Erythroderma Erythroderma Work up 1) Exam for pertinent positives and negatives: • lymphadenopathy • primary skin lesions (i.e. nail pits of psoriasis) • mucosal involvement • Hepatosplenomagaly 2) laboratory • Chem 7, LFT, CBC • HIV • Multiple biopsies over time 3) review of medications 4) age-appropriate malignancy screening 5) evaluate hemodynamic stability Erythroderma Management 1) -

Febrile Illness with Skin Rashes Jin Han Kang Department of Pediatrics, College of Medicine, the Catholic University of Korea, Seoul, Korea
Review Article Infection & http://dx.doi.org/10.3947/ic.2015.47.3.155 Infect Chemother 2015;47(3):155-166 Chemotherapy ISSN 2093-2340 (Print) · ISSN 2092-6448 (Online) Febrile Illness with Skin Rashes Jin Han Kang Department of Pediatrics, College of Medicine, The Catholic University of Korea, Seoul, Korea Skin rashes that appear during febrile illnesses are in fact caused by various infectious diseases. Since infectious exanthema- tous diseases range from mild infections that disappear naturally to severe infectious diseases, focus on and basic knowledge of these diseases is very important. But, these include non-infectious diseases, so that comprehensive knowledge of these oth- er diseases is required. Usually, early diagnostic testing for a febrile illness with a rash is inefficient. For clinical diagnosis of diseases accompanied by skin rash and fever, a complete history must be taken, including recent travel, contact with animals, medications, and exposure to forests and other natural environments. In addition, time of onset of symptoms and the character- istics of the rash itself (morphology, location, distribution) could be helpful in the clinical diagnosis. It is also critical to under- stand the patient’s history of specific underlying diseases. However, diagnostic basic tests could be helpful in diagnosis if they are repeated and the clinical course is monitored. Generally, skin rashes are nonspecific and self-limited. Therefore, it could be clinically meaningful as a characteristic diagnostic finding in a very small subset of specific diseases. Key Words: Febrile illness; Skin rash; Infectious disease; Non-infectious disease Introduction non-infectious diseases, so that comprehensive knowledge of these other diseases is required for clinical diagnosis of a fe- When patients with febrile illnesses also develop a rash, they brile illness with a rash. -
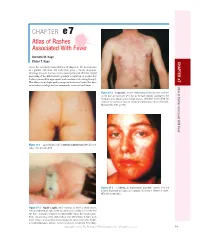
CHAPTER E7 Atlas of Rashes Associated with Fever
CHAPTER e7 Atlas of Rashes Associated With Fever Kenneth M. Kaye Elaine T. Kaye CHAPTER e7 Given the extremely broad differential diagnosis, the presentation of a patient with fever and rash often poses a thorny diagnostic challenge for even the most astute and experienced clinician. Rapid narrowing of the differential by prompt recognition of a rash’s key features can result in appropriate and sometimes life-saving therapy. This atlas presents high-quality images of a variety of rashes that have an infectious etiology and are commonly associated with fever. Atlas of Rashes Associated With Fever Figure e7-3 In measles, discrete erythematous lesions become confluent on the face and neck over 2–3 days as the rash spreads downward to the trunk and arms, where lesions remain discrete. (Reprinted from K Wolff, RA Johnson: Color Atlas & Synopsis of Clinical Dermatology, 5th ed. New York, McGraw-Hill, 2005, p 788.) Figure e7-1 Lacy reticular rash of erythema infectiosum (fifth disease) caused by parvovirus B19. Figure e7-4 In rubella, an erythematous exanthem spreads from the hairline downward and clears as it spreads. (Courtesy of Stephen E. Gellis, MD; with permission.) Figure e7-2 Koplik’s spots, which manifest as white or bluish lesions with an erythematous halo on the buccal mucosa, usually occur in the first two days of measles symptoms and may briefly overlap the measles exan- them. The presence of the erythematous halo differentiates Koplik’s spots from Fordyce’s spots (ectopic sebaceous glands), which occur in the mouths of healthy individuals. (Source: Centers for Disease Control and Prevention.) Copyright © 2012 The McGraw-Hill Companies, Inc. -

Childhood Rashes That Present to the ED Part I: Viral and Bacterial Issues
March 2007 Childhood Rashes That Volume 4, Number 3 Author Present To The ED Part I: Viral Marc S. Lampell, MD University of Rochester School of Medicine and And Bacterial Issues Dentistry, Assistant Professor of Emergency Medicine, Assistant Professor of Pediatrics You’re managing to keep your head above water during the evening shift in the Peer Reviewers pediatric emergency department of a university hospital when you get a phone call Sharon Mace, MD Associate Professor, Emergency Department, Ohio from a local physician who’s sending you a dilemma that she’s been struggling State University School of Medicine, Director of with for three days. The case is a four-year-old girl who recently moved from Pediatric Education And Quality Improvement and Russia (unknown vaccination status) with persistent fever over 40oC for three Director of Observation Unit, Cleveland Clinic, Faculty, MetroHealth Medical Center, Emergency Medicine days. The child is “toxic” appearing and has impressive coryza and a non-produc- Residency tive cough. Her eyes are very red with scant non-purulent discharge for which the pediatrician prescribed antibiotic drops. The child developed a rather impressive Paula J. Whiteman, MD non-pruritic facial rash that rapidly spread to the trunk. Despite a negative “rapid Medical Director, Pediatric Emergency Medicine, Encino-Tarzana Regional Medical Center; strep” test and blood and urine cultures, the pediatrician’s concern for this “toxic” Attending Physician, Cedars-Sinai Medical Center, kid was sufficiently worrisome that she elected to start antibiotics. In the pediatric Los Angeles, CA ED, the child is noted to be normotensive, but is tachycardic (consistent with her CME Objectives febrile state). -

Infectious Diseases of Gambia
INFECTIOUS DISEASES OF GAMBIA Stephen Berger, MD 2018 Edition Infectious Diseases of Gambia Copyright Infectious Diseases of Gambia - 2018 edition Stephen Berger, MD Copyright © 2018 by GIDEON Informatics, Inc. All rights reserved. Published by GIDEON Informatics, Inc, Los Angeles, California, USA. www.gideononline.com Cover design by GIDEON Informatics, Inc No part of this book may be reproduced or transmitted in any form or by any means without written permission from the publisher. Contact GIDEON Informatics at [email protected]. ISBN: 978-1-4988-1775-2 Visit www.gideononline.com/ebooks/ for the up to date list of GIDEON ebooks. DISCLAIMER Publisher assumes no liability to patients with respect to the actions of physicians, health care facilities and other users, and is not responsible for any injury, death or damage resulting from the use, misuse or interpretation of information obtained through this book. Therapeutic options listed are limited to published studies and reviews. Therapy should not be undertaken without a thorough assessment of the indications, contraindications and side effects of any prospective drug or intervention. Furthermore, the data for the book are largely derived from incidence and prevalence statistics whose accuracy will vary widely for individual diseases and countries. Changes in endemicity, incidence, and drugs of choice may occur. The list of drugs, infectious diseases and even country names will vary with time. Scope of Content Disease designations may reflect a specific pathogen (ie, Adenovirus infection), generic pathology (Pneumonia - bacterial) or etiologic grouping (Coltiviruses - Old world). Such classification reflects the clinical approach to disease allocation in the Infectious Diseases Module of the GIDEON web application. -

Infectious Diseases in Critical Care Medicine
Infectious Diseases in Critical Care Medicine Cunha_978-1420092400_TP.indd 1 10/5/09 4:21:18 PM INFECTIOUS DISEASE AND THERAPY Series Editor Burke A. Cunha Winthrop-University Hospital Mineola, New York and State University of New York School of Medicine Stony Brook, New York 1. Parasitic Infections in the Compromised Host, edited by Peter D. Walter and Robert M. Genta 2. Nucleic Acid and Monoclonal Antibody Probes: Applications in Diagnostic Method- ology, edited by Bala Swaminathan and Gyan Prakash 3. Opportunistic Infections in Patients with the Acquired Immunodeficiency Syndrome, edited by Gifford Leoung and John Mills 4. Acyclovir Therapy for Herpesvirus Infections, edited by David A. Baker 5. The New Generation of Quinolones, edited by Clifford Siporin, Carl L. Heifetz, and John M. Domagala 6. Methicillin-Resistant Staphylococcus aureus: Clinical Management and Laboratory Aspects, edited by Mary T. Cafferkey 7. Hepatitis B Vaccines in Clinical Practice, edited by Ronald W. Ellis 8. The New Macrolides, Azalides, and Streptogramins: Pharmacology and Clinical Applications, edited by Harold C. Neu, Lowell S. Young, and Stephen H. Zinner 9. Antimicrobial Therapy in the Elderly Patient, edited by Thomas T. Yoshikawa and Dean C. Norman 10. Viral Infections of the Gastrointestinal Tract: Second Edition, Revised and Expanded, edited by Albert Z. Kapikian 11. Development and Clinical Uses of Haemophilus b Conjugate Vaccines, edited by Ronald W. Ellis and Dan M. Cranoff 12. Pseudomonas aeruginosa Infections and Treatment, edited by Aldona L. Battch and Raymond P. Smith 13. Herpesvirus Infections, edited by Ronald Glaser and James F. Jones 14. Chronic Fatigue Syndrome, edited by Stephen E. -

Infectious Diseases of Tanzania
INFECTIOUS DISEASES OF TANZANIA Stephen Berger, MD Infectious Diseases of Tanzania - 2014 edition Infectious Diseases of Tanzania - 2014 edition Stephen Berger, MD Copyright © 2014 by GIDEON Informatics, Inc. All rights reserved. Published by GIDEON Informatics, Inc, Los Angeles, California, USA. www.gideononline.com Cover design by GIDEON Informatics, Inc No part of this book may be reproduced or transmitted in any form or by any means without written permission from the publisher. Contact GIDEON Informatics at [email protected]. ISBN: 978-1-4988-0208-6 Visit http://www.gideononline.com/ebooks/ for the up to date list of GIDEON ebooks. DISCLAIMER Publisher assumes no liability to patients with respect to the actions of physicians, health care facilities and other users, and is not responsible for any injury, death or damage resulting from the use, misuse or interpretation of information obtained through this book. Therapeutic options listed are limited to published studies and reviews. Therapy should not be undertaken without a thorough assessment of the indications, contraindications and side effects of any prospective drug or intervention. Furthermore, the data for the book are largely derived from incidence and prevalence statistics whose accuracy will vary widely for individual diseases and countries. Changes in endemicity, incidence, and drugs of choice may occur. The list of drugs, infectious diseases and even country names will vary with time. Scope of Content Disease designations may reflect a specific pathogen (ie, Adenovirus infection), generic pathology (Pneumonia - bacterial) or etiologic grouping (Coltiviruses - Old world). Such classification reflects the clinical approach to disease allocation in the Infectious Diseases Module of the GIDEON web application.