Suspected Trigeminal Nerve Neuropathy Causing Persistent
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Atypical & Idiopathic Facial Pain
ATYPICAL & IDIOPATHIC FACIAL PAIN Definition According to the International Association for the Study of Pain (IASP), chronic facial pain refers to symptoms which have been present for at least 6 months. ‘Atypical’ pain is a diagnosis of exclusion after other conditions have been considered and eliminated (i.e. it is idiopathic) and is characterized by chronic, constant pain in the absence of any apparent cause in the face or brain. Many information sources suggest that all ‘unexplained’ facial pains are termed Atypical Facial Pain but this is not the case. Categories of idiopathic facial pain conditions include Neuropathic Pain due to sensory nerve damage, Chronic Regional Pain Syndrome (CRPS) from sympathetic nerve damage and Atypical Facial Pain. Atypical odontalgia, or phantom tooth pain is a variation of atypical facial pain where intense discomfort is centered around a tooth or group of teeth with no obvious dental or oral disease. Epidemiology Atypical facial pain is more common in women than in men; most patients attending a facial pain clinic are women aged between 30 and 50 years. Although any area of the face can be involved, the most commonly affected area is the maxillary region. In the majority of patients there is no disease or other cause found. In a few patients the symptoms represent serious disease. In a small number of patients the pain may be one consequence of significant psychological or psychiatric disease. Clinical presentation Atypical facial pain is very variable in its presentation. Often it is characterized by continuous, daily pain of variable intensity. Typically, the pain is deep and poorly localized, is described as dull and aching, and does not waken the patient from sleep. -

Zeroing in on the Cause of Your Patient's Facial Pain
Feras Ghazal, DDS; Mohammed Ahmad, Zeroing in on the cause MD; Hussein Elrawy, DDS; Tamer Said, MD Department of Oral Health of your patient's facial pain (Drs. Ghazal and Elrawy) and Department of Family Medicine/Geriatrics (Drs. Ahmad and Said), The overlapping characteristics of facial pain can make it MetroHealth Medical Center, Cleveland, Ohio difficult to pinpoint the cause. This article, with a handy at-a-glance table, can help. [email protected] The authors reported no potential conflict of interest relevant to this article. acial pain is a common complaint: Up to 22% of adults PracticE in the United States experience orofacial pain during recommendationS F any 6-month period.1 Yet this type of pain can be dif- › Advise patients who have a ficult to diagnose due to the many structures of the face and temporomandibular mouth, pain referral patterns, and insufficient diagnostic tools. disorder that in addition to Specifically, extraoral facial pain can be the result of tem- taking their medication as poromandibular disorders, neuropathic disorders, vascular prescribed, they should limit disorders, or atypical causes, whereas facial pain stemming activities that require moving their jaw, modify their diet, from inside the mouth can have a dental or nondental cause and minimize stress; they (FIGURE). Overlapping characteristics can make it difficult to may require physical therapy distinguish these disorders. To help you to better diagnose and and therapeutic exercises. C manage facial pain, we describe the most common causes and underlying pathological processes. › Consider prescribing a tricyclic antidepressant for patients with persistent idiopathic facial pain. C Extraoral facial pain Extraoral pain refers to the pain that occurs on the face out- 2-15 Strength of recommendation (SoR) side of the oral cavity. -

Hereditary Hypertrophic Neuropathy Combining Features of Tic Douloureux, Charcot- Marie-Tooth Disease, and Deafness
Hereditary hypertrophic neuropathy combining features of tic douloureux, Charcot- Marie-Tooth disease, and deafness Hereditary hypertrophic sensorimotor poly- neuropathy combining the features of Charcot- Marie-Tooth disease, trigeminal neuralgia, and Robert P. Cruse, D.O. deafness occurred through four generations of John P. Conomy, M.D. a family originating in Haywood County, North Asa J. Wilbourn, M.D. Carolina. Fourteen individuals had pes cavus, Maurice R. Hanson, M.D. distal muscle atrophy, depressed or absent mus- cle stretch reflexes, cutaneous sensory deficits, Department of Neurology and defective proprioception. Six family mem- bers had recurrent, lancinating, trigeminal pain, and seven were deaf. The family was brought under scrutiny when the propositus, a 60-year-old woman, was examined for treat- ment of tic douloureux. Neurologic informa- tion was ultimately obtained regarding 52 family members. No history of consanguinity could be ascertained within this kinship. The genealogy of the family we studied is presented in Figure 1. In addition to clinical neurologic examinations, electromyographic and nerve conduction stud- ies were obtained on those individuals indicated in the genealogy diagram. Audiometric studies were obtained when there was clinical indication of defective hearing. Quantitative cutaneous sensory testing was obtained in the propositus. 107 Downloaded from www.ccjm.org on October 1, 2021. For personal use only. All other uses require permission. 108 Cleveland Clinic Quarterly Vol. 44, No. 3 ' • CMT a TIC Deaf CMT He Examined MS f Died O Not Affected 0 D led before age onset (O Multiple normal d1 ond j si be. -1—i Two Marriages —Indicates number of slbs. -

Molecular Diagnostics of Charcot-Marie-Tooth Disease and Related Peripheral Neuropathies
17_Lupski 3/30/06 1:47 PM Page 243 NeuroMolecular Medicine Copyright © 2006 Humana Press Inc. All rights of any nature whatsoever reserved. ISSN0895-8696/06/08:243–254/$30.00 doi: 10.1385/NMM:8:1:243 REVIEW ARTICLE Molecular Diagnostics of Charcot-Marie-Tooth Disease and Related Peripheral Neuropathies Kinga Szigeti,1 Eva Nelis,2,3 and James R. Lupski*,1,4 1Departments of Molecular and Human Genetics, Baylor College of Medicine, Houston, TX; 2Molecular Genetics, Flanders Interuniversity Institute for Biotechnology, Institute Born-Bunge, University of Antwerp, Antwerpen, Belgium; 3Laboratory of Neurogenetics, Institute Born-Borge, University of Antwerp, Antwerpen, Belgium; and 4Pediatrics, Baylor College of Medicine, and Texas Children Hospital, Houston, TX 77030 Received January 10, 2006; Revised January 13, 2006; Accepted January 13, 2006 Abstract DNAdiagnostics plays an important role in the characterization and management of patients manifesting inherited peripheral neuropathies. We describe the clinical integration of molecular diagnostics with medical history, physical examination, and electrophysiological studies. Mole- cular testing can help establish a secure diagnosis, enable genetic counseling regarding recurrence risk, potentially provide prognostic information, and in the near future may be important for the choice of therapies. doi: 10.1385/NMM:8:1:243 Index Entries:Molecular diagnostics; Charcot-Marie-Tooth disease; CMT; hereditary neuropathy with liability to pressure palsies; HNPP; Dejerine-Sottas neuropathy; DSN; congenital hypomyelinating neuropathy; CHN; CMT1A duplication; HNPP deletion. Introduction genetic testing, one needs to be familiar with the diagnostic tests available, choose the appropriate Molecular genetic diagnosis has become an inte- patients for testing, and utilize the diagnostic tools gral part of the evaluation of patients with hered- in a logical fashion to optimize the use of resources. -

Chronic Orofacial Pain: Burning Mouth Syndrome and Other Neuropathic
anagem n M e ai n t P & f o M l e Journal of a d n i c r i u n o e J Pain Management & Medicine Tait et al., J Pain Manage Med 2017, 3:1 Review Article Open Access Chronic Orofacial Pain: Burning Mouth Syndrome and Other Neuropathic Disorders Raymond C Tait1, McKenzie Ferguson2 and Christopher M Herndon2 1Saint Louis University School of Medicine, St. Louis, USA 2Southern Illinois University Edwardsville School of Pharmacy, Edwardsville, USA *Corresponding author: RC Tait, Department of Psychiatry, Saint Louis University School of Medicine,1438 SouthGrand, Boulevard, St Louis, MO-63104, USA, Tel: 3149774817; Fax: 3149774879; E-mail: [email protected] Recevied date: October 4, 2016; Accepted date: January 17, 2017, Published date: January 30, 2017 Copyright: © 2017 Raymond C Tait, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Chronic orofacial pain is a symptom associated with a wide range of neuropathic, neurovascular, idiopathic, and myofascial conditions that affect a significant proportion of the population. While the collective impact of the subset of the orofacial pain disorders involving neurogenic and idiopathic mechanisms is substantial, some of these are relatively uncommon. Hence, patients with these disorders can be vulnerable to misdiagnosis, sometimes for years, increasing the symptom burden and delaying effective treatment. This manuscript first reviews the decision tree to be followed in diagnosing any neuropathic pain condition, as well as the levels of evidence needed to make a diagnosis with each of several levels of confidence: definite, probable, or possible. -

Trigeminal Isolated Sensory Neuropathy (TISN) and FOSMN
Cruccu et al. BMC Neurology (2014) 14:248 DOI 10.1186/s12883-014-0248-2 RESEARCH ARTICLE Open Access Trigeminal isolated sensory neuropathy (TISN) and FOSMN syndrome: despite a dissimilar disease course do they share common pathophysiological mechanisms? Giorgio Cruccu1*, Elena M Pennisi2, Giovanni Antonini3, Antonella Biasiotta1, Giulia di Stefano1, Silvia La Cesa1, Caterina Leone1, Salvatore Raffa4, Claudia Sommer5 and Andrea Truini1 Abstract Background: Patients presenting with bilateral trigeminal hypoesthesia may go on to have trigeminal isolated sensory neuropathy, a benign, purely trigeminal neuropathy, or facial-onset sensory motor neuronopathy (FOSMN), a malignant life-threatening condition. No diagnostic criteria can yet differentiate the two conditions at their onset. Nor is it clear whether the two diseases are distinct entities or share common pathophysiological mechanisms. Methods: Seeking pathophysiological and diagnostic information to distinguish these two conditions at their onset, in this neurophysiological and morphometric study we neurophysiologically assessed function in myelinated and unmyelinated fibres and histologically examined supraorbital nerve biopsy specimens with optic and electron microscopy in 13 consecutive patients with recent onset trigeminal hypoesthesia and pain. Results: The disease course distinctly differed in the 13 patients. During a mean 10 year follow-up whereas in eight patients the disease remained relatively stable, in the other five it progressed to possibly life-threatening motor disturbances and extra-trigeminal spread. From two to six years elapsed between the first sensory symptoms and the onset of motor disorders. In patients with trigeminal isolated sensory neuropathy (TISN) and in those with FOSMN neurophysiological and histological examination documented a neuronopathy manifesting with trigeminal nerve damage selectively affecting myelinated fibres, but sparing the Ia-fibre-mediated proprioceptive reflex. -

Neuropathic Orofacial Pain the Brochure Is Provided Compliments Of
Neuropathic_Pain_Brochure_Neuropathic_Pain_Brochure 6/9/2010 11:41 AM Page 1 Neuropathic orofacial paiN The brochure is provided compliments of This brochure in intended for informational purposes only and should be considered a replacement for a professional treatment for a health care professional. To locate knowledgeable and experienced expert in orofacial pain, contact: The American Academy of Orofacial Pain 174 S. New York Ave. POB 478 Oceanville, NJ 08231 P: 609-504-1311 E: [email protected] W: www.aaop.org To locate knowledgeable and experienced expert in Trigeminal Neuralgia, contact: Trigeminal Neuralgia Association 2801 SW Archer Road Gainesville, FL 32608 P: 352-376-9955 E: [email protected] W: www.tna-support.org Neuropathic_Pain_Brochure_Neuropathic_Pain_Brochure 6/9/2010 11:41 AM Page 3 coNteNts 1-Neuropathic orofacial paiN 4-GettiNG help/What to expect 6-commoN Neuropathic orofacial paiN DisorDers aND their treatmeNt 6 - triGem iNal NeuralGia 8 - pre-triGem iNal NeuralGia 9 - atypical oDoNtalGia (phaNtom tooth paiN) 10 - chroNic reGioNal paiN syNDrome 12 - iN coNclusioN Neuropathic_Pain_Brochure_Neuropathic_Pain_Brochure 6/9/2010 11:41 AM Page 4 Neuropathic orofacial paiN Of the many pains that can effect the head and neck, perhaps the most confusing and difficult to diagnose are a group of maladies called Neuropathic Orofacial Pain Disorders. These neuropathic pain disorders are often chronic and arise from the brain and nerves of the head, face and neck. If you have experienced the frustration of having a toothache or face pain and, after seeing many doctors, still don't know where the pain is coming from, Brain you may be suffering from a neuropathic pain Spinal Cord disorder. -

Trigeminal Neuralgia – Diagnosis and Treatment
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Archivio della ricerca- Università di Roma La Sapienza Review Cephalalgia 2017, Vol. 37(7) 648–657 ! International Headache Society 2017 Trigeminal neuralgia – Reprints and permissions: sagepub.co.uk/journalsPermissions.nav diagnosis and treatment DOI: 10.1177/0333102416687280 journals.sagepub.com/home/cep Stine Maarbjerg1, Giulia Di Stefano2, Lars Bendtsen1 and Giorgio Cruccu2 Abstract Introduction: Trigeminal neuralgia (TN) is characterized by touch-evoked unilateral brief shock-like paroxysmal pain in one or more divisions of the trigeminal nerve. In addition to the paroxysmal pain, some patients also have continuous pain. TN is divided into classical TN (CTN) and secondary TN (STN). Etiology and pathophysiology: Demyelination of primary sensory trigeminal afferents in the root entry zone is the predominant pathophysiological mechanism. Most likely, demyelination paves the way for generation of ectopic impulses and ephaptic crosstalk. In a significant proportion of the patients, the demyelination is caused by a neurovascular conflict with morphological changes such as compression of the trigeminal root. However, there are also other unknown etiological factors, as only half of the CTN patients have morphological changes. STN is caused by multiple sclerosis or a space-occupying lesion affecting the trigeminal nerve. Differential diagnosis and treatment: Important differential diagnoses include trigeminal autonomic cephalalgias, posttraumatic or postherpetic pain and other facial pains. First line treatment is prophylactic medication with sodium channel blockers, and second line treatment is neurosurgical intervention. Future perspectives: Future studies should focus on genetics, unexplored etiological factors, sensory function, the neurosurgical outcome and complications, combination and neuromodulation treatment as well as development of new drugs with better tolerability. -

1 United States District Court Eastern District Of
UNITED STATES DISTRICT COURT EASTERN DISTRICT OF TENNESSEE AT CHATTANOOGA JOHANNA DETERDING, ) ) Plaintiff, ) ) v. ) Case No:1:11-CV-13 ) Collier/Carter MICHAEL S. ASTRUE, ) Commissioner of Social Security, ) ) Defendant. ) REPORT AND RECOMMENDATION This action was instituted pursuant to 42 U.S.C. '' 405(g) and 1383(c)(3) seeking judicial review of the final decision of the Commissioner denying the plaintiff a period of disability, disability insurance benefits, and supplemental security income under Title II and Title XVI of the Social Security Act, 42 U.S.C. '' 416(I), 423, and 1382. 1 This matter has been referred to the undersigned pursuant to 28 U.S.C. ' 636(b) and Rule 72(b) of the Federal Rules of Civil Procedure for a report and recommendation regarding the disposition of plaintiff's motion for judgment on the pleadings (Doc. 11) and defendant=s motion for summary judgment (Doc. 17 ). For the reasons stated herein, I RECOMMENDED the Commissioner=s decision be REVERSED and REMANDED pursuant to Sentence Four of 42 U.S.C. ' 405(g). 1Since the relevant DIB and SSI regulations cited herein are virtually identical, citations will only be made to the DIB regulations, found at 20 C.F.R. '' 404.1500-404.1599. The parallel SSI regulations are found at 20 C.F.R. '' 416.900-416.999, corresponding to the last two digits of the DIB cites (e.g., 20 C.F.R. ' 404.1545 corresponds with 20 C.F.R. ' 416.945). 1 Case 1:11-cv-00013-CLC-WBC Document 19 Filed 02/02/12 Page 1 of 25 PageID #: <pageID> + Plaintiff's Age, Education, and Past Work Experience Plaintiff had a high-school education, along with three years of college, and was 40 years old at the time of her February 2009 hearing (Tr. -
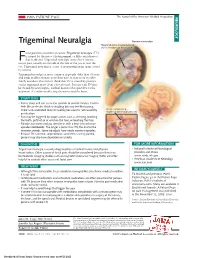
Trigeminal Neuralgia Trigeminal Neuralgia Trigeminal Nerve (Cranial Nerve V) and Its 3 Regions of Innervation Acial Pain Has a Number of Causes
JAMA PATIENT PAGE The Journal of the American Medical Association NEUROLOGY Trigeminal Neuralgia Trigeminal neuralgia Trigeminal nerve (cranial nerve V) and its 3 regions of innervation acial pain has a number of causes. Trigeminal neuralgia (TN) is named for the nerve (the trigeminal, or fifth cranial nerve) Fthat is affected. Trigeminal neuralgia causes brief, intense, BRAIN severe pain, usually on one side of the face or the jaw or near the V eye. Trigeminal neuralgia is a type of neuropathic pain (pain caused 1 by nerves). V V 2 Trigeminal neuralgia is more common in people older than 50 years 3 and tends to affect women more than men. It may occur in other family members. Researchers think that TN is caused by pressure on the trigeminal nerve from a blood vessel. Persons with TN may be treated by neurologists, medical doctors who specialize in the treatment of conditions affecting the nerves and the brain. BRAIN SYMPTOMS • Pain is sharp and can last a few seconds to several minutes. It often V feels like an electric shock or stabbing but may feel like burning. 1 There is no associated fever or swelling because it is not caused by Artery compressing trigeminal nerve and branches an infection. • Pain may be triggered by simple actions such as chewing, brushing V2 the teeth, puffs of air or wind on the face, or touching the face. V V 3 • Episodes can come and go, sometimes with a long time between 3 episodes (remission). The longer a person has TN, the shorter the V1 remission periods. -

A Misdiagnosis of Atypical Trigeminal Neuralgia
RESIDENT & FELLOW SECTION Clinical Reasoning: A misdiagnosis of atypical trigeminal neuralgia Jaclyn R. Duvall, MD, and Carrie E. Robertson, MD Correspondence Dr. Robertson Neurology 2019;93:124-131. doi:10.1212/WNL.0000000000007790 ® [email protected] Section 1 A 47-year-old man presented with right-sided facial pain that started 2 years prior. He described the pain as extremely intense, stabbing along the right jaw, lasting 5–60 seconds. This pain was exacerbated by chewing, and to a lesser degree, by brushing his teeth. The pain was so intense that he avoided eating when possible, leading to a 20-pound weight loss. When he did eat, he would try to chew on the left side of his mouth. Around the onset of these symptoms, he also noticed a persistent numbness and burning extending from the right lower earlobe to the lateral angle of the jaw that was exacerbated by turning his head to the right. The patient was given a diagnosis of atypical trigeminal neuralgia (TN) and sent to our headache clinic for further management. Questions for consideration: 1. What features are typical and atypical for classical TN? 2. What is your differential diagnosis in this patient presenting with facial pain? GO TO SECTION 2 From the Department of Neurology, Headache Division, Mayo Clinic, Rochester, MN. Go to Neurology.org/N for full disclosures. Funding information and disclosures deemed relevant by the authors, if any, are provided at the end of the article. 124 Copyright © 2019 American Academy of Neurology Copyright © 2019 American Academy of Neurology. Unauthorized reproduction of this article is prohibited. -

Osteopetrosis Associated with Familial Paraplegia: Report of a Family
Paraplegia (1975), 13, 143-152 OSTEOPETROSIS ASSOCIATED WITH FAMILIAL PARAPLEGIA: REPORT OF A FAMILY By SKIP JACQUES*, M.D., JOHN T. GARNER, M. D., DAVID JOHNSON, M.D. and C. HUNTER SHELDEN, M. D. Departments of Neurosurgery and Radiology, Huntington Memorial Hospital, Pasadena, Ca., and the Huntington Institute of Applied Medical Research, Pasadena, Ca., U.S.A. Abstract. A clinical analysis of three members of a family with documented osteopetrosis and familial paraplegia is presented. All patients had a long history of increased bone density and slowly progressing paraparesis of both legs. A thorough review of the literature has revealed no other cases which presented with paraplegia without spinal cord com pression. Although the etiologic factor or factors remain unknown, our review supports the contention that this is a distinct clinical entity. IN 1904, a German radiologist, Heinrich Albers-Schonberg, described a 26-year old man with multiple fractures and generalised sclerosis of the skeleton. The disease has henceforth commonly been known as Albers-Schonberg disease or marble osteopetrosis, a term first introduced by Karshner in 1922. Other eponyms are bone disease, osteosclerosis fragilis generalisata, and osteopetrosis generalisata. Approximately 300 cases had been reported in the literature by 1968. It has been generally accepted that the disease presents in two distinct forms, an infantile progressive disease and a milder form in childhood and adolescence. The two forms differ clinically and genetically. A dominant pattern of inheritance is usually seen in the benign type whereas the severe infantile form is usually inherited as a Mendelian recessive. This important distinction has not been well emphasised.