Electrical Engineering Units and Constants
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Programming Guideline for S7-1200/S7-1500 STEP 7 and STEP 7 Safety in TIA Portal
Background and System Description 03/2017 Programming Guideline for S7-1200/S7-1500 STEP 7 and STEP 7 Safety in TIA Portal http://www.siemens.com/simatic-programming-guideline Warranty and Liability Warranty and Liability Note The Application Examples are not binding and do not claim to be complete regarding the circuits shown, equipping and any eventuality. The Application Examples do not represent customer-specific solutions. They are only intended to provide support for typical applications. You are responsible for ensuring that the described products are used correctly. These Application Examples do not relieve you of the responsibility to use safe practices in application, installation, operation and maintenance. When using these Application Examples, you recognize that we cannot be made liable for any damage/claims beyond the liability clause described. We reserve the right to make changes to these Application Examples at any time without prior notice. If there are any deviations between the recommendations provided in these Application Examples and other Siemens publications – e.g. Catalogs – the contents of the other documents have priority. We do not accept any liability for the information contained in this document. Any claims against us – based on whatever legal reason – resulting from the use of the examples, information, programs, engineering and performance data etc., described in this Application Example shall be excluded. Such an exclusion shall not apply in the case of mandatory liability, e.g. under the German Product Liability Act (“Produkthaftungsgesetz”), in case of intent, gross negligence, or injury of life, body or health, guarantee for the quality of a product, fraudulent concealment of a deficiency or breach of a condition which goes to the root of the contract (“wesentliche Vertragspflichten”). -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Physical Chemistry LD
Physical Chemistry LD Electrochemistry Chemistry Electrolysis Leaflets C4.4.5.2 Determining the Faraday constant Aims of the experiment To perform an electrolysis. To understand redox reactions in practice. To work with a Hoffman electrolysis apparatus. To understand Faraday’s laws. To understand the ideal gas equation. The second law is somewhat more complex. It states that the Principles mass m of an element that is precipitated by a specific When a voltage is applied to a salt or acid solution, material amount of charge Q is proportional to the atomic mass, and is migration occurs at the electrodes. Thus, a chemical reaction inversely proportional to its valence. Stated more simply, the is forced to occur through the flow of electric current. This same amount of charge Q from different electrolytes always process is called electrolysis. precipitates the same equivalent mass Me. The equivalent mass Me is equal to the molecular mass of an element divid- Michael Faraday had already made these observations in the ed by its valence z. 1830s. He coined the terms electrolyte, electrode, anode and cathode, and formulated Faraday's laws in 1834. These count M M = as some of the foundational laws of electrochemistry and e z describe the relationships between material conversions In order to precipitate this equivalent mass, 96,500 Cou- during electrochemical reactions and electrical charge. The lombs/mole are always needed. This number is the Faraday first of Faraday’s laws states that the amount of moles n, that constant, which is a natural constant based on this invariabil- are precipitated at an electrode is proportional to the charge ity. -

The Laby Experiment
Anna Binnie The Laby Experiment Abstract After J.J. Thomson demonstrated that the atom was not an indivisible entity but contained negatively charged corpuscles, the search for the determination of magnitude of this fundamental unit of electric charge commenced. This paper will briefly discuss the different attempts to measure this quantity of unit charge e. It will outline the progress of these attempts and will focus on the little known work of Australian, Thomas Laby, who attempted to reconcile the value of e determined from two very different approaches. This was probably the last attempt to measure e and was made at a time that most of the scientific world was busy with other issues. The measurement of the unit electric charge e, by timing the fall of charged oil drops is an experiment that has been performed by generations of undergraduate physics students. The experiment is often termed the Millikan oil drop experiment after R.A. Millikan (1868-1953) who conceived and performed it during the period 1907-1917. This experiment was repeated in 1939-40 by V.D. Hopper (1913b), lecturer in Natural Philosophy at Melbourne University and T.H. Laby (1880-1946) who was Professor. As far as we know this was the last time that the experiment was performed “seriously”, i.e. performed carefully by professional physicists and fully reported in major journals. The question arises who was Laby and why did he conduct this experiment such along time after Millikan has completed his work? Thomas Laby was born in 1880 at Creswick Victoria. In 1883 the family moved to New South Wales where young Thomas was educated. -

The International System of Units (SI)
NAT'L INST. OF STAND & TECH NIST National Institute of Standards and Technology Technology Administration, U.S. Department of Commerce NIST Special Publication 330 2001 Edition The International System of Units (SI) 4. Barry N. Taylor, Editor r A o o L57 330 2oOI rhe National Institute of Standards and Technology was established in 1988 by Congress to "assist industry in the development of technology . needed to improve product quality, to modernize manufacturing processes, to ensure product reliability . and to facilitate rapid commercialization ... of products based on new scientific discoveries." NIST, originally founded as the National Bureau of Standards in 1901, works to strengthen U.S. industry's competitiveness; advance science and engineering; and improve public health, safety, and the environment. One of the agency's basic functions is to develop, maintain, and retain custody of the national standards of measurement, and provide the means and methods for comparing standards used in science, engineering, manufacturing, commerce, industry, and education with the standards adopted or recognized by the Federal Government. As an agency of the U.S. Commerce Department's Technology Administration, NIST conducts basic and applied research in the physical sciences and engineering, and develops measurement techniques, test methods, standards, and related services. The Institute does generic and precompetitive work on new and advanced technologies. NIST's research facilities are located at Gaithersburg, MD 20899, and at Boulder, CO 80303. -

CAR-ANS Part 5 Governing Units of Measurement to Be Used in Air and Ground Operations
CIVIL AVIATION REGULATIONS AIR NAVIGATION SERVICES Part 5 Governing UNITS OF MEASUREMENT TO BE USED IN AIR AND GROUND OPERATIONS CIVIL AVIATION AUTHORITY OF THE PHILIPPINES Old MIA Road, Pasay City1301 Metro Manila UNCOTROLLED COPY INTENTIONALLY LEFT BLANK UNCOTROLLED COPY CAR-ANS PART 5 Republic of the Philippines CIVIL AVIATION REGULATIONS AIR NAVIGATION SERVICES (CAR-ANS) Part 5 UNITS OF MEASUREMENTS TO BE USED IN AIR AND GROUND OPERATIONS 22 APRIL 2016 EFFECTIVITY Part 5 of the Civil Aviation Regulations-Air Navigation Services are issued under the authority of Republic Act 9497 and shall take effect upon approval of the Board of Directors of the CAAP. APPROVED BY: LT GEN WILLIAM K HOTCHKISS III AFP (RET) DATE Director General Civil Aviation Authority of the Philippines Issue 2 15-i 16 May 2016 UNCOTROLLED COPY CAR-ANS PART 5 FOREWORD This Civil Aviation Regulations-Air Navigation Services (CAR-ANS) Part 5 was formulated and issued by the Civil Aviation Authority of the Philippines (CAAP), prescribing the standards and recommended practices for units of measurements to be used in air and ground operations within the territory of the Republic of the Philippines. This Civil Aviation Regulations-Air Navigation Services (CAR-ANS) Part 5 was developed based on the Standards and Recommended Practices prescribed by the International Civil Aviation Organization (ICAO) as contained in Annex 5 which was first adopted by the council on 16 April 1948 pursuant to the provisions of Article 37 of the Convention of International Civil Aviation (Chicago 1944), and consequently became applicable on 1 January 1949. The provisions contained herein are issued by authority of the Director General of the Civil Aviation Authority of the Philippines and will be complied with by all concerned. -

VBII Safety Switches Selection and Application Guide
VBII Safety Switches Selection and Application Guide usa.siemens.com/safetyswitches You asked for it. Siemens listened. Siemens asked contractors for everything they wanted in an enclosed safety switch. Their input helped create the toughest, most reliable, most hassle-free enclosed safety switch in the business – the Siemens Type VBII Safety Switch. It’s a switch that’s right for any commercial, industrial or special use application. The Siemens Safety Switch line offers a list of important features that gives contractors a competitive edge: Highly visible, easy-to-grip red handle Ratings Visible blade construction 30-1200 amps Door that opens greater than 180º 240 and 600 volts AC Quick-make, quick-break mechanism 250 and 600 volts DC 200% optional neutrals (100-600 Amps) 100 AlC for general duty switches All copper current-carrying parts on 200 AlC for heavy duty switches heavy duty switches (except lugs) Design E horsepower rated Positive two- and three-point mounting Suitable for use as service equipment Provisions for UL Class T, R, J, L and H fuses 12X overload rating that exceeds industry standard of 10X Contents Features 4-5 Hub and lug data 26-27 Enclosure ratings and types 6-10 Dimensions special application Plug fuse type 11 safety switches 28 General duty switches features 12 Double throw switches 29-30 General duty types 13 Detailed dimension drawings 31-59 Heavy duty switches features 14-15 Replacement parts 60 Heavy duty switch types 16-18 Fuse application and selection 61 Heavy duty switches 4X&12 Fuse application and dimensions 62-63 with viewing window 19 Ratings and test requirements 64-65 Special application/ Suggested specifications 66-67 Interlocked receptacle switches 20-22 Catalog numbering system 68 Accessories 23-25 2 3 One tough switch: Siemens Type VBII Safety Switch Siemens now offers a complete line of enclosed switches featuring unique and innovative designs that are unparalleled in the industry. -

Series 81000™ 720 Ampere Vacuum Controller
™ Instructions Series 81000 Installation 720 Ampere Vacuum Controller Operation Type 96H6 Maintenance (Distribution Voltage 2400-4800 VAC; Utilization Voltage 2300-4600 VAC) SGIM-9098B DANGER Hazardous voltages. Will cause death, serious personal injury or equipment or property damage. Always de-energize and ground the equipment before maintenance. Read and understand this instruction manual before installing, operating, or maintaining the equipment. Maintenance should be performed only by qualified personnel. The use of unauthorized parts in the repair of the equipment or tampering by unqualified personnel may result in dangerous conditions which may cause death or serious personal injury or equipment or property damage. Follow all safety instructions contained herein. IMPORTANT The information contained herein is general in nature and not intended for specific application purposes. It does not relieve the user of responsibility to use sound practices in application, installation, operation, and maintenance of the equipment purchased. Siemens reserves the right to make changes in the specifications shown herein or to make improvements at any time without notice or obligations. Should a conflict arise between the general information contained in this publication and the contents of drawings or supplementary material or both, the latter shall take precedence. QUALIFIED PERSON For the purpose of this manual and product labels a qualified person is one who is familiar with the installation, construction, operation, or maintenance of the equipment and the hazards involved. In addition, this person has the following qualifications: (a) is trained and authorized to energize, de-energize, clear, ground, and tag circuits and equipment in accordance with established safety practices. (b) is trained in the proper care and use of protective equipment such as rubber gloves, hard hat, safety glasses or face shields, flash clothing, etc., in accordance with established safety practices. -

New Best Estimates of the Values of the Fundamental Constants
ing a vicious cycle in of the base units of our system of measurement, such which developing as the kilogram, metre, second, ampere, and kelvin— countries that lag in the base units of the SI, the International System of S&T capacity fall fur- units. The Consultative Committee for Units (of the ther behind, as International Committee on Weights and Measures, industrialized BIPM/CCU) advises the Comité International des nations with financial Poids et Mesures on defining (or redefining) each of resources and a the base units of the SI in terms of the fundamental trained scientific constants rather than in terms of material artefacts or work force exploit time intervals related to the rotation of the earth. new knowledge and Today, some, but not all, of the base units of the SI are technologies more defined in this way, and we are working on the quickly and inten- remainder. sively. These deficits can leave entire developing In December 2003, new best estimates of the fun- economies behind. And when nations need to damental constants were released. These are com- respond to diseases such as HIV or SARS, or make piled and published with the authority of a CODATA decisions about issues such as stem-cell research or committee that exists for this purpose, but in practice genetically modified foods, this lack of S&T infrastruc- they are produced (on this occasion) by Barry Taylor ture can breed unfounded fear and social discord. and Peter Mohr at the U.S. National Institute for The report asserts that there is no reason why, in an Standards and Technology (NIST), in Gaithersburg, era in which air travel and the Internet already tightly MD. -

Experiment 21A FARADAY's
Experiment 21A FV 11/15/11 FARADAY’S LAW MATERIALS: DPDT (double pole/double throw) switch, digital ammeter, J-shaped platinum electrode and holder, power supply, carbon electrode, electrical lead, 10 mL graduated cylinder, 100 mL beaker, 400 mL beaker, buret, thermometer, starch solution, 0.20 M KI, 1 M H2SO4, 0.0200 M Na2S2O3. PURPOSE: The purpose of this experiment is to determine values for the Faraday constant and Avogadro’s number. LEARNING OBJECTIVES: By the end of this experiment, the student should be able to demonstrate the following proficiencies: 1. Construct an electrolytic cell from a diagram. 2. Determine the number of moles of products formed in a redox reaction from experimental data. 3. Determine the total charge that has passed through an electrolytic cell. 4. Calculate values for the Faraday constant and Avogadro’s number from experimental data. DISCUSSION: Forcing an electrical current through an electrolytic cell can cause a nonspontaneous chemical reaction to occur. For example, when direct current is passed through a solution of aqueous potassium iodide, KI, the following reactions occur at the electrodes: − − anode: 2 I (aq) → I2 (aq) + 2 e (oxidation) − − cathode: 2 H2O (l) + 2 e → H2 (g) + 2 OH (aq) (reduction) − − overall redox: 2 I (aq) + 2 H2O (l) → I2 (aq) + H2 (g) + 2 OH (aq) Electrons can be treated stoichiometrically like the other chemical species in these reactions. Thus, the number of moles of products formed is related to the number of moles of electrons that pass through the cell during the electrolysis. Iodine is formed at the anode in this electrolysis and dissolves in the solution upon stirring.1 Hydrogen gas is formed at the cathode and will be collected in an inverted graduated cylinder by displacement of water. -
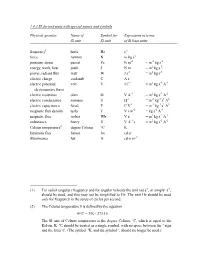
1.4.3 SI Derived Units with Special Names and Symbols
1.4.3 SI derived units with special names and symbols Physical quantity Name of Symbol for Expression in terms SI unit SI unit of SI base units frequency1 hertz Hz s-1 force newton N m kg s-2 pressure, stress pascal Pa N m-2 = m-1 kg s-2 energy, work, heat joule J N m = m2 kg s-2 power, radiant flux watt W J s-1 = m2 kg s-3 electric charge coulomb C A s electric potential, volt V J C-1 = m2 kg s-3 A-1 electromotive force electric resistance ohm Ω V A-1 = m2 kg s-3 A-2 electric conductance siemens S Ω-1 = m-2 kg-1 s3 A2 electric capacitance farad F C V-1 = m-2 kg-1 s4 A2 magnetic flux density tesla T V s m-2 = kg s-2 A-1 magnetic flux weber Wb V s = m2 kg s-2 A-1 inductance henry H V A-1 s = m2 kg s-2 A-2 Celsius temperature2 degree Celsius °C K luminous flux lumen lm cd sr illuminance lux lx cd sr m-2 (1) For radial (angular) frequency and for angular velocity the unit rad s-1, or simply s-1, should be used, and this may not be simplified to Hz. The unit Hz should be used only for frequency in the sense of cycles per second. (2) The Celsius temperature θ is defined by the equation θ/°C = T/K - 273.15 The SI unit of Celsius temperature is the degree Celsius, °C, which is equal to the Kelvin, K. -

The International System of Units (SI) - Conversion Factors For
NIST Special Publication 1038 The International System of Units (SI) – Conversion Factors for General Use Kenneth Butcher Linda Crown Elizabeth J. Gentry Weights and Measures Division Technology Services NIST Special Publication 1038 The International System of Units (SI) - Conversion Factors for General Use Editors: Kenneth S. Butcher Linda D. Crown Elizabeth J. Gentry Weights and Measures Division Carol Hockert, Chief Weights and Measures Division Technology Services National Institute of Standards and Technology May 2006 U.S. Department of Commerce Carlo M. Gutierrez, Secretary Technology Administration Robert Cresanti, Under Secretary of Commerce for Technology National Institute of Standards and Technology William Jeffrey, Director Certain commercial entities, equipment, or materials may be identified in this document in order to describe an experimental procedure or concept adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the entities, materials, or equipment are necessarily the best available for the purpose. National Institute of Standards and Technology Special Publications 1038 Natl. Inst. Stand. Technol. Spec. Pub. 1038, 24 pages (May 2006) Available through NIST Weights and Measures Division STOP 2600 Gaithersburg, MD 20899-2600 Phone: (301) 975-4004 — Fax: (301) 926-0647 Internet: www.nist.gov/owm or www.nist.gov/metric TABLE OF CONTENTS FOREWORD.................................................................................................................................................................v