Use of Dna Sequencing to Identify the Origin of Northwestern and Southwestern Pond Turtles in Captive Breeding Programs
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

1875 El Prado San Diego, CA 92101 (619) 238-1233
Volunteer Orientation Handbook 1875 El Prado San Diego, CA 92101 (619) 238-1233 www.rhfleet.org The Reuben H. Fleet Science Center seeks to inspire lifelong learning by furthering the public understanding and enjoyment of science and technology. Welcome Welcome to the Volunteer and Internship Programs at the Reuben H. Fleet Science Center. We thank you for your interest in helping our organization inspire lifelong learning in our community. We sincerely hope that your experiences here will be rewarding, educational and fun! Volunteers and interns play an integral role in the operation of the Fleet and its programs. Our team is made up of over 200 dedicated volunteers and interns (and roughly 100 employees) serving in a multitude of roles, all helping to spark a better understanding and enjoyment of science and technology by the public. We would like to express our sincere appreciation for your interest in our volunteer and intern programs. We couldn't do any of this without your support. THANK YOU for sharing your time and talent with us! General Information Mission Statement: The Reuben H. Fleet Science Center seeks to inspire lifelong learning by furthering the public understanding and enjoyment of science and technology. Physical Address: 1875 El Prado (at the intersection of Park Blvd. & Space Theater Way) San Diego, CA 92101 Mailing Address: PO Box 33303 San Diego, CA 92163 Telephone: (619) 238-1233 Website: www.rhfleet.org Hours: Open every day, including holidays! We open every day at 10:00 a.m. (exception: 11:30 a.m. on Christmas day). Closing times vary—check our website for updates. -
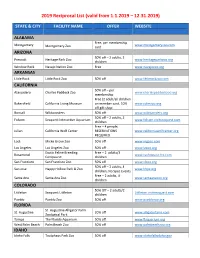
Reciprocal Zoo List 2019 for Website
2019 Reciprocal List (valid from 1.1.2019 – 12.31.2019) STATE & CITY FACILITY NAME OFFER WEBSITE ALABAMA Free, per membership Montgomery www.montgomeryzoo.com Montgomery Zoo card ARIZONA 50% off – 2 adults, 3 Prescott Heritage Park Zoo www.heritageparkzoo.org children Window Rock Navajo Nation Zoo Free www.navajozoo.org ARKANSAS Little Rock Little Rock Zoo 50% off www.littlerockzoo.com CALIFORNIA 50% off – per Atascadero Charles Paddock Zoo www.charlespaddockzoo.org membership Free (2 adult/all children Bakersfield California Living Museum on member card, 10% www.calmzoo.org off gift shop Bonsall Wildwonders 50% off www.wildwonders.org 50% off – 2 adults, 2 Folsom Seaquest Interactive Aquarium www.folsom.visitseaquest.com children Free – 4 people; Julian California Wolf Center RESERVATIONS www.californiawolfcenter.org REQUIRED Lodi Micke Grove Zoo 50% off www.mgzoo.com Los Angeles Los Angeles Zoo 50% off www.lazoo.org Exotic Feline Breeding Free – 2 adults/3 Rosamond www.cathopuise.fcc.com Compound children San Francisco San Francisco Zoo 50% off www.sfzoo.org 50% off – 2 adults, 4 San Jose Happy Hollow Park & Zoo www.hhpz.org children, No Spec Events Free – 2 adults, 4 Santa Ana Santa Ana Zoo www.santaanazoo.org children COLORADO 50% Off – 2 adults/2 Littleton Seaquest Littleton Littleton.visitseaquest.com children Pueblo Pueblo Zoo 50% off www.pueblozoo.org FLORIDA St. Augustine Alligator Farm St. Augustine 20% off www.alligatorfarm.com Zoological Park Tampa The Florida Aquarium 50% off www.flaquarium.org West Palm Beach Palm Beach Zoo 50% off www.palmbeachzoo.org IDAHO Idaho Falls Tautphaus Park Zoo 50% off www.idahofallsidaho.gov 2019 Reciprocal List (valid from 1.1.2019 – 12.31.2019) Free – 2 adults, 5 Pocatello Pocatello Zoo www.zoo.pocatello.us children ILLINOIS Free – 2 adults, 3 Springfield Henson Robinson Zoo children. -

Casa Del Prado in Balboa Park
Chapter 19 HISTORY OF THE CASA DEL PRADO IN BALBOA PARK Of buildings remaining from the 1915 Panama-California Exposition, exhibit buildings north of El Prado in the agricultural section survived for many years. They were eventually absorbed by the San Diego Zoo. Buildings south of El Prado were gone by 1933, except for the New Mexico and Kansas Buildings. These survive today as the Balboa Park Club and the House of Italy. This left intact the Spanish-Colonial complex along El Prado, the main east-west avenue that separated north from south sections The Sacramento Valley Building, at the head of the Plaza de Panama in the approximate center of El Prado, was demolished in 1923 to make way for the Fine Arts Gallery. The Southern California Counties Building burned down in 1925. The San Joaquin Valley and the Kern-Tulare Counties Building, on the promenade south of the Plaza de Panama, were torn down in 1933. When the Science and Education and Home Economy buildings were razed in 1962, the only 1915 Exposition buildings on El Prado were the California Building and its annexes, the House of Charm, the House of Hospitality, the Botanical Building, the Electric Building, and the Food and Beverage Building. This paper will describe the ups and downs of the 1915 Varied Industries and Food Products Building (1935 Food and Beverage Building), today the Casa del Prado. When first conceived the Varied Industries and Food Products Building was called the Agriculture and Horticulture Building. The name was changed to conform to exhibits inside the building. -

Balboa Park Facilities
';'fl 0 BalboaPark Cl ub a) Timken MuseumofArt ~ '------___J .__ _________ _J o,"'".__ _____ __, 8 PalisadesBuilding fDLily Pond ,------,r-----,- U.,..p_a_s ..,.t,..._---~ i3.~------ a MarieHitchcock Puppet Theatre G BotanicalBuild ing - D b RecitalHall Q) Casade l Prado \ l::..-=--=--=---:::-- c Parkand Recreation Department a Casadel Prado Patio A Q SanD iegoAutomot iveMuseum b Casadel Prado Pat io B ca 0 SanD iegoAerospace Museum c Casadel Prado Theate r • StarlightBow l G Casade Balboa 0 MunicipalGymnasium a MuseumofPhotograph icArts 0 SanD iegoHall of Champions b MuseumofSan Diego History 0 Houseof PacificRelat ionsInternational Cottages c SanDiego Mode l RailroadMuseum d BalboaArt Conservation Cente r C) UnitedNations Bui lding e Committeeof100 G Hallof Nations u f Cafein the Park SpreckelsOrgan Pavilion 4D g SanDiego Historical Society Research Archives 0 JapaneseFriendship Garden u • G) CommunityChristmas Tree G Zoro Garden ~ fI) ReubenH.Fleet Science Center CDPalm Canyon G) Plaza deBalboa and the Bea Evenson Fountain fl G) HouseofCharm a MingeiInternationa l Museum G) SanDiego Natural History Museum I b SanD iegoArt I nstitute (D RoseGarden j t::::J c:::i C) AlcazarGarden (!) DesertGarden G) MoretonBay Ag T ree •........ ••• . I G) SanDiego Museum ofMan (Ca liforniaTower) !il' . .- . WestGate (D PhotographicArts Bui lding ■ • ■ Cl) 8°I .■ m·■ .. •'---- G) CabrilloBridge G) SpanishVillage Art Center 0 ... ■ .■ :-, ■ ■ BalboaPar kCarouse l ■ ■ LawnBowling Greens G 8 Cl) I f) SeftonPlaza G MiniatureRail road aa a Founders'Plaza Cl)San Diego Zoo Entrance b KateSessions Statue G) War MemorialBuil ding fl) MarstonPoint ~ CentroCu lturalde la Raza 6) FireAlarm Building mWorld Beat Cultura l Center t) BalboaClub e BalboaPark Activ ity Center fl) RedwoodBrid geCl ub 6) Veteran'sMuseum and Memo rial Center G MarstonHouse and Garden e SanDiego American Indian Cultural Center andMuseum $ OldG lobeTheatre Comp lex e) SanDiego Museum ofArt 6) Administration BuildingCo urtyard a MayS. -

APPLIED NUTRITIONAL STUDIES with ZOOLOGICAL REPTILES by KYLE SAMUEL THOMPSON Bachelor of Science in Animal Science California S
APPLIED NUTRITIONAL STUDIES WITH ZOOLOGICAL REPTILES By KYLE SAMUEL THOMPSON Bachelor of Science in Animal Science California State University Fresno Fresno, California 2006 Master of Science in Animal Science Oklahoma State University Stillwater, Oklahoma 2011 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of DOCTOR OF PHILOSOPHY May, 2016 APPLIED NUTRITIONAL STUDIED WITH ZOOLOGICAL REPTILES Dissertation Approved: Dr. Clint Krehbiel Dissertation Adviser Dr. Gerald Horn Dr. Scott Carter Dr. Lionel Dawson ii ACKNOWLEDGEMENTS “Until one has loved an animal, a part of one's soul remains unawakened." -Anatole France First and foremost, I would like to thank my Lord and Savior, Jesus Christ. He has always provided a light for me during times of discouragement. Secondly I would like to give a very big thank you to my advisor and mentor Dr. Clint Krehbiel who has been very patient and caring all these years. Thank you for all the guidance and giving me the freedom to pursue my dreams. I also want to extend a thank you to Donna Perry, Diana Batson, and Debra Danley for always being there for me to comfort and laugh. I would like to send a special thank you to the San Diego Zoo Nutrition Services team, Dr. Mike Schlegel, Edith Galindo and Michele Gaffney. Thank you for your guidance and patience and continued friendship. Further thank you is needed to Dr. Schlegel for accepting me in 2009 and opening my eyes to the world of zoo and captive wildlife nutrition. -
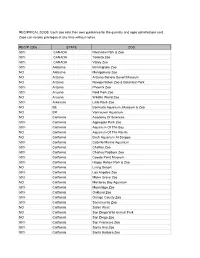
2006 Reciprocal List
RECIPRICAL ZOOS. Each zoo sets their own guidelines for the quantity and ages admitted per card. Zoos can revoke privileges at any time without notice. RECIP 2006 STATE ZOO 50% CANADA Riverview Park & Zoo 50% CANADA Toronto Zoo 50% CANADA Valley Zoo 50% Alabama Birmingham Zoo NO Alabama Montgomery Zoo NO Arizona Arizona-Sonora Desert Museum NO Arizona Navajo Nation Zoo & Botanical Park 50% Arizona Phoenix Zoo 50% Arizona Reid Park Zoo NO Arizona Wildlife World Zoo 50% Arkansas Little Rock Zoo NO BE Bermuda Aquarium, Museum & Zoo NO BR Vancouver Aquarium NO California Academy Of Sciences 50% California Applegate Park Zoo 50% California Aquarium Of The Bay NO California Aquarium Of The Pacific NO California Birch Aquarium At Scripps 50% California Cabrillo Marine Aquarium 50% California Chaffee Zoo 50% California Charles Paddock Zoo 50% California Coyote Point Museum 50% California Happy Hollow Park & Zoo NO California Living Desert 50% California Los Angeles Zoo 50% California Micke Grove Zoo NO California Monterey Bay Aquarium 50% California Moonridge Zoo 50% California Oakland Zoo 50% California Orange County Zoo 50% California Sacramento Zoo NO California Safari West NO California San Diego Wild Animal Park NO California San Diego Zoo 50% California San Francisco Zoo 50% California Santa Ana Zoo 50% California Santa Barbara Zoo NO California Seaworld San Diego 50% California Sequoia Park Zoo NO California Six Flags Marine World NO California Steinhart Aquarium NO CANADA Calgary Zoo 50% Colorado Butterfly Pavilion NO Colorado Cheyenne -

North American Zoos with Mustelid Exhibits
North American Zoos with Mustelid Exhibits List created by © birdsandbats on www.zoochat.com. Last Updated: 19/08/2019 African Clawless Otter (2 holders) Metro Richmond Zoo San Diego Zoo American Badger (34 holders) Alameda Park Zoo Amarillo Zoo America's Teaching Zoo Bear Den Zoo Big Bear Alpine Zoo Boulder Ridge Wild Animal Park British Columbia Wildlife Park California Living Museum DeYoung Family Zoo GarLyn Zoo Great Vancouver Zoo Henry Vilas Zoo High Desert Museum Hutchinson Zoo 1 Los Angeles Zoo & Botanical Gardens Northeastern Wisconsin Zoo & Adventure Park MacKensie Center Maryland Zoo in Baltimore Milwaukee County Zoo Niabi Zoo Northwest Trek Wildlife Park Pocatello Zoo Safari Niagara Saskatoon Forestry Farm and Zoo Shalom Wildlife Zoo Space Farms Zoo & Museum Special Memories Zoo The Living Desert Zoo & Gardens Timbavati Wildlife Park Turtle Bay Exploration Park Wildlife World Zoo & Aquarium Zollman Zoo American Marten (3 holders) Ecomuseum Zoo Salomonier Nature Park (atrata) ZooAmerica (2.1) 2 American Mink (10 holders) Bay Beach Wildlife Sanctuary Bear Den Zoo Georgia Sea Turtle Center Parc Safari San Antonio Zoo Sanders County Wildlife Conservation Center Shalom Wildlife Zoo Wild Wonders Wildlife Park Zoo in Forest Park and Education Center Zoo Montana Asian Small-clawed Otter (38 holders) Audubon Zoo Bright's Zoo Bronx Zoo Brookfield Zoo Cleveland Metroparks Zoo Columbus Zoo and Aquarium Dallas Zoo Denver Zoo Disney's Animal Kingdom Greensboro Science Center Jacksonville Zoo and Gardens 3 Kansas City Zoo Houston Zoo Indianapolis -

USS Midway Museum Historic Gaslamp Quarter Balboa Park
Approx. 22 Miles Approx. 28 Miles San Diego Zoo Del Mar Legoland Fairgrounds Safari Park Del Mar Beaches DOG FRIENDLY 56 North Beach 5 Torrey Pines State Natural Reserve Hiking Torrey Pines Golf Course 805 Torrey Pines Gliderport University of California San Diego Birch Aquarium at Scripps Westfield UTC Mall La Jolla Shores La Jolla Cove 52 Village of La Jolla SeaWorld USS Midway Historic Gaslamp Balboa Park & Museum Quarter San Diego Zoo Approx. 12 Miles Approx. 15 Miles Approx. 16 Miles Approx. 16 Miles Fun Things To Do Within Walking Distance Torrey Pines Golf Course (0.5 mi) – Perfect your swing at the world renowned Torrey Pines Golf Course, home to two 18-hole championship courses. This public course has a driving range and is open every day until 30 minutes before dusk. Call our Golf Team at 1-800-991-GOLF (4653) to book your tee time. Torrey Pines State Natural Reserve (0.8 mi) – Hike a trail in this beautiful 2,000-acre coastal state park overlooking the Pacific Ocean. Some trails lead directly to Torrey Pines State Beach. Trail maps available at our Concierge Desk. Torrey Pines Gliderport (1.5 mi) – Visit North America's top paragliding and hang gliding location and try an instructional tandem flight. Please call ahead since all flights are dependent on the wind conditions - (858) 452-9858. Fun Things To Do Just a Short Drive Away La Jolla Playhouse (2 mi) – A not-for-profit, professional theatre at the University of California San Diego. See Concierge for current showings. Birch Aquarium (3 mi) – Experience stunning sea life at Birch Aquarium at Scripps Institute of Oceanography. -

The San Diego Zoo Safari Park
Welcome to the San Diego Zoo Safari Park Please note the Africa Tram is not included with school admission tickets. You must have purchased a separate Africa Tram ticket to experience this attraction. Here are a couple of tips to help you make the most of your day. Chaperones, please share these insider tips with your students. 1. Chaperones, please stay with your students at all times. The Safari Park is a large and exciting place and it is easy to get separated. Lost chaperones are brought to Ranger Base (indicated on reverse) to meet up with your students. 2. For best viewing of our animals, please use low voices, do not bang on the glass and try not to run. The animals will move away or hide from loud noise and fast movements. 3. If you want to feed our animals, please visit Lorikeet Landing (from 10:00 A.M. to 3:45 P.M. Lorikeet food is available for purchase, 10 students at a time), The other animals are on special diets and people food might make them sick. 4. If you want to pet our animals, please visit our Petting Kraal. Your teacher must schedule a visit time if you are visiting October 1st- 31st and May 11th - April 23rd. 5. Have a good time, take lots of pictures and ask questions. Safari Park staff are happy to assist. To further enhance your visit, you may want to see one of our shows or meet one of our animal ambassadors up close by seeing an Animal Encounter. -

2017 Reciprocal List
2017 Reciprocal List In order to expand your membership experience, The Zoo in Forest Park and Education Center has partnered with zoos, aquariums, museums, and parks across the United States to offer discounted admission to its traveling members for 2017. As reciprocity status is subject to change, please call ahead to verify pricing and hours. Be prepared to show current membership card and valid photo ID at all locations. Traveling members are subject to all rules and regulations set by participating locations. For more information regarding your membership and membership benefits, please contact Nicholas Kinsman at (413) 733-2251, ext. 304, or [email protected] Arizona Orange County Zoo – (714) 973-6841 Heritage Park Zoological Sanctuary – 1 Irvine Park Road (928) 778-4242 Orange, CA 92862 1403 Heritage Park Road Discount: 100% Prescott, AZ 86301 - Excludes special events Discount: 50% - Does not include parking - Excludes special events Santa Ana Zoo – (714) 953-8555 Reid Park Zoo – (520) 881-4753 1801 E. Chestnut Avenue 3400 E Zoo Court Santa Ana, CA 92701 Tucson, AZ 85716 Discount: 100% Discount: 50% - Discount applies to regular daily admission Connecticut - Excludes special events Beardsley Zoo – (203) 394-6565 California 1875 Noble Avenue Bridgeport, CT 06610 Micke Grove Zoo – (209) 331-2010 Discount: 100% 11793 N. Micke Grove Road - Covers admission of 2 adults & up to 6 children Lodi, CA 95240 - Parking is free Discount: 100% Florida Happy Hollow Park & Zoo – (408) 794-6444 1300 Senter Road St. Augustine Alligator Farm - (904) 842-3337 San Jose, CA 95112 999 Anastasia Boulevard Discount: 50% St. Augustine, FL 32080 Discount: 50% Big Bear Alpine Zoo – (909) 878-4200 43285 Goldmine Drive Illinois Big Bear Lake, CA 92315 Discount: 50% Cosely Zoo - (630) 665-5534 - Valid for 2 adults & up to 2 children 1356 N. -

RECIPROCAL LIST: January 1, 2019 - December 31, 2019 to Visit a Participating Zoo, Present Your Membership Cards and Picture Ids at the Admission Gate
Gladys Porter Zoo - RECIPROCAL LIST: January 1, 2019 - December 31, 2019 To visit a participating zoo, present your membership cards and picture IDs at the admission gate. THE NUMBER OF VISITORS ADMITTED AND ANY DISCOUNTS VARY DEPENDING ON THE ZOO OR AQUARIUM and are subject to change without notice. Remember, because this list changes throughout the year, we suggest that you call ahead before you plan to visit any particular zoo. State/City Institution Discount Phone # Notes & other benefits CANADA Calgary-Alberta Calgary Zoo 50% (403)232-9300 Toronto Toronto Zoo 50% (416)392-5900 Granby-Quebec Granby Zoo 50% (450) 372-9113 x2103 A:2, Ch: 2; No plus MEXICO Leon Parque Zoologico de Leon 50% 52 (477) 210-2335 x102 50% Off Safari Tour; $2 Parking Singapore Sentosa Island S.E.A. Aquarium 50% (656)577-8669 Sentosa Island Dolphin Island 50% (656)577-8669 Alabama Birmingham Birmingham Zoo 50% (205) 879-0409 x248 Montgomery Montgomery Zoo Mann Wildlife Learning Muse Free (334)625-4900 Alaska Seward Alaska SeaLife Center 50% (907)224-6355 Arizona Phoenix The Phoenix Zoo 50% (602) 914-4393 Tempe SEA LIFE Arizona Aquarium 50% (877) 526-3960 Tucson Reid Park Zoo 50% (520) 327-6343 Arkansas Little Rock Little Rock Zoo 50% (501) 661-7218 A: 2, Ch:All listed under 18 yrs; Plus; $2 Parking California Atascadero Charles Paddock Zoo Free (805) 461-5080 x2105 A: 2, Ch: 2; No plus; Free parking Eureka Sequoia Park Zoo 50% (707) 441-4263 A: 2, Ch: All listed under 18 yrs.; No plus; Free parking Fresno Fresno Chaffee Zoo 50% (559) 498-5921 A: 2, Ch: 2; No -

Overview of the Acorn Group, Inc. Blending the Skills of Planners
Overview of The Acorn Group, Inc. Blending the skills of planners, designers, and educators, The Acorn Group offers award-winning services in interpretive planning and design. Part art, part science, accented with storytelling and exquisite design, our interpretive master plans, exhibits, panels, and programs create sensory-rich experiences that bring your content to life. Established in 1990, The Acorn Group is dedicated to the field of interpretation and actively in- volved in the National Association for Interpretation, as well as other professional organizations. Our clients are diverse, ranging from government agencies to private and non-profit institutions. Project sites include interpretive centers, ecological reserves, museums, botanical gardens, zoos, parklands, and educational institutions. Our greatest satisfaction comes from seeing plans and drawings become reality and watching visitors take delight in new experiences. The Acorn Group’s projects and efforts have been recognized nationally, receiving such awards as the Exhibit Design Award, Print and Media Award, and Interpretive Media Award by the National Association for Interpretation, Award of Excellence by the American Society of Landscape Archi- tects, Best of Show by the Western Fairs Association, Award of Excellence by the California Parks and Recreation Society, and National Education Award by the Association of Zoos and Aquariums. The Orange County League of Conservation Voters presented The Acorn Group with the Orange County Environmental Business of the Year Award in 2005. In 2007, The Acorn Group received the First Place Interpretive Media Award from the National Association for Interpretation for design of Nix Nature Center. In 2012, the North American Association for Environment Education presented The Acorn Group and Acorn Naturalists with their Outstanding Service Award.