Utilizing LC/UV and LC/MS for the Characterization, Isolation, And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
(12) Patent Application Publication (10) Pub. No.: US 2013/0315843 A1 HAUGHT Et Al
US 2013 0315843A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0315843 A1 HAUGHT et al. (43) Pub. Date: Nov. 28, 2013 (54) COMPOSITION FOR REDUCTION OF TRPA1 Related U.S. Application Data AND TRPV1 SENSATIONS (60) Provisional application No. 61/652,035, filed on May (71) Applicant: The Procter & Gamble Company, 25, 2012, provisional application No. 61/682,887, (US) filed on Aug. 14, 2012. Publication Classification (72) Inventors: John Christian HAUGHT, West Chester, OH (US); Koti (51) Int. Cl. SREEKRISHNA, Mason, OH (US); A618/42 (2006.01) Sourav DAS, Kolkata (IN); Steve A618/35 (2006.01) Hamilton HOKE, II, West Chester, OH A61O 11/00 (2006.01) (US); Timothy Woodrow A618/37 (2006.01) COFFINDAFFER, Maineville, OH A61O5/10 (2006.01) (US); Katharine Anne BAKES, A618/30 (2006.01) Cincinnati, OH (US); William Michael (52) U.S. Cl. GLANDORF, Mason, OH (US) CPC. A61K 8/42 (2013.01); A61O 5/10 (2013.01); A61K 8/30 (2013.01); A61O II/00 (2013.01); (73) Assignee: The Procter & Gamble Company, A61K 8/37 (2013.01); A61K 8/35 (2013.01) Cincinnati, OH (US) USPC ...................... 424/48; 435/29: 8/406; 424/53 (57) ABSTRACT (21) Appl. No.: 13/873,749 A personal care composition and method of using a personal care composition having menthol and/or hydrogen peroxide (22) Filed: Apr. 30, 2013 and a TRPA1 and/or TRPV1 receptor antagonists. US 2013/03 15843 A1 Nov. 28, 2013 COMPOSITION FOR REDUCTION OF TRPA1 able as a positive signal of efficacy. Further, Some of these AND TRPV1 SENSATIONS molecules may exhibit the ability to reduce sulfur and amine species present in the body in the form of Michael Acceptors FIELD OF THE INVENTION (Yoshida et al., Tetrahedron Letters, 51:5134-5136 (2010)). -
Capsicum Oleoresin and Homocapsaicin
Printed on: Wed Jan 06 2021, 02:44:36 AM Official Status: Currently Official on 06-Jan-2021 DocId: 1_GUID-1560FD9B-BE0F-495E-9994-C5718733DB4C_2_en-US (EST) Printed by: Jinjiang Yang Official Date: Official as of 01-May-2019 Document Type: USP @2021 USPC 1 nordihydrocapsaicin, nonivamide, decanylvanillinamide, Capsicum Oleoresin and homocapsaicin. DEFINITION ASSAY Capsicum Oleoresin is an alcoholic extract of the dried ripe · CONTENT OF TOTAL CAPSAICINOIDS fruits of Capsicum. It contains NLT 6.5% of total Mobile phase: A mixture of acetonitrile and diluted capsaicinoids, calculated as the sum of capsaicin, phosphoric acid (1 in 1000) (2:3) dihydrocapsaicin, nordihydrocapsaicin, nonivamide, Standard solution A: 0.2 mg/mL of USP Capsaicin RS in decanylvanillinamide, and homocapsaicin, all calculated on methanol the anhydrous basis. The nonivamide content is NMT 5% of Standard solution B: 0.1 mg/mL of USP the total capsaicinoids, calculated on the anhydrous basis. Dihydrocapsaicin RS in methanol [CAUTIONÐCapsicum Oleoresin is a powerful irritant, and Sample solution: 5 mg/mL of Capsicum Oleoresin in even in minute quantities produces an intense burning methanol. Pass a portion of this solution through a filter of sensation when it comes in contact with the eyes and 0.2-µm pore size, and use the filtrate as the Sample solution. tender parts of the skin. Care should be taken to protect Chromatographic system the eyes and to prevent contact of the skin with (See Chromatography á621ñ, System Suitability.) Capsicum Oleoresin.] Mode: LC IDENTIFICATION -

Product Nutritional Analysis
Product Nutritional Analysis CA Nutri Chemical Analysis Analysis Unit Price (ex VAT) SANAS Accredited? Lead Time Energy by Calculation kJ or kcal No charge if part of Full Nutri Carbohydrate by Calculation g/100g No charge if part of Full Nutri Moisture g/100g R 193 2 Ash g/100g R 193 3 Protein g/100g R 355 3 Glycaemic Carbohydrates g/100g R 1 649 5 Total Sugars g/100g R 1 088 5 (Glucose, Fructose, Sucrose, Lactose and Maltose) Total Fat by AOAC 996.06 g/100g R 950 7 Full Nutritional Fatty acid Composition by AOAC 996.06 g/100g R 1 087 Yes Label of which Saturated g/100g of which Monounsaturated g/100g of which Polyunsaturated g/100g Included as part of Fatty Acid 7 of which Trans Fatty Acids g/100g Composition Omega 3 mg/100g Omega 6 mg/100g Cholesterol mg/100g R 842 7 Total Dietary Fibre by AOAC 985.29 g/100g R 1 636 8 to 12 Sodium mg/100g R 529 5 Salt calculated from Sodium results g/100g No charge if Sodium requested Yes Salt Salt by chloride titration g/100g R 662 Yes 5 Acid Insoluble Ash g/100g R 390 5 to 7 Crude Fibre g/100g R 1 374 Yes 5 to 7 Water Activity - R 527 7 Other pH - R 150 2 Density g/ml R 133 Yes 3 Caffeine mg/100g R 705 Yes 5 Total Capsaicinoids mg/kg R 665 5 (Capsaicin, dihydrocapsaicin, nordihydrocapsaicin and Scoville Heat Value) Antimony (Sb) mg/kg R 813 Arsenic (As) mg/kg R 813 Subc to SGS 10 Calcium (Ca) mg/100g R 529 Chromium (Cr) mg/kg R 813 Copper (Cu) mg/kg R 529 Yes 5 to 7 Iron (Fe) mg/kg R 529 Yes 5 Inorganic Food Testing Potassium (K) mg/100g R 529 Yes 5 Phosphorus (P) mg/kg R 813 Subc 10 Selenium (Se) -

TRP Channel Transient Receptor Potential Channels
TRP Channel Transient receptor potential channels TRP Channel (Transient receptor potential channel) is a group of ion channels located mostly on the plasma membrane of numerous human and animal cell types. There are about 28 TRP channels that share some structural similarity to each other. These are grouped into two broad groups: Group 1 includes TRPC ("C" for canonical), TRPV ("V" for vanilloid), TRPM ("M" for melastatin), TRPN, and TRPA. In group 2, there are TRPP ("P" for polycystic) and TRPML ("ML" for mucolipin). Many of these channels mediate a variety of sensations like the sensations of pain, hotness, warmth or coldness, different kinds of tastes, pressure, and vision. TRP channels are relatively non-selectively permeable to cations, including sodium, calcium and magnesium. TRP channels are initially discovered in trp-mutant strain of the fruit fly Drosophila. Later, TRP channels are found in vertebrates where they are ubiquitously expressed in many cell types and tissues. TRP channels are important for human health as mutations in at least four TRP channels underlie disease. www.MedChemExpress.com 1 TRP Channel Inhibitors, Antagonists, Agonists, Activators & Modulators (-)-Menthol (E)-Cardamonin Cat. No.: HY-75161 ((E)-Cardamomin; (E)-Alpinetin chalcone) Cat. No.: HY-N1378 (-)-Menthol is a key component of peppermint oil (E)-Cardamonin ((E)-Cardamomin) is a novel that binds and activates transient receptor antagonist of hTRPA1 cation channel with an IC50 potential melastatin 8 (TRPM8), a of 454 nM. Ca2+-permeable nonselective cation channel, to 2+ increase [Ca ]i. Antitumor activity. Purity: >98.0% Purity: 99.81% Clinical Data: Launched Clinical Data: No Development Reported Size: 10 mM × 1 mL, 500 mg, 1 g Size: 10 mM × 1 mL, 5 mg, 10 mg, 25 mg, 50 mg, 100 mg (Z)-Capsaicin 1,4-Cineole (Zucapsaicin; Civamide; cis-Capsaicin) Cat. -

Determination of Capsaicinoid Profile of Some Peppers Sold in Nigerian Markets
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2014, 6(4):648-654 ISSN : 0975-7384 Research Article CODEN(USA) : JCPRC5 Determination of capsaicinoid profile of some peppers sold in Nigerian markets 1N. C. Nwokem *, 2C. O. Nwokem, 2Y. O. Usman, 1O. J. Ocholi, 2M. L. Batari and 3A. A. Osunlaja 1Department of Chemistry, Ahmadu Bello University, Zaria, Nigeria 2National Research Institute for Chemical Technology, Zaria, Nigeria 3Umar Suleiman College of Education, Gashua, Nigeria ____________________________________________________________________________________________ ABSTRACT The capsaicinoid profile of six different peppers sold in Nigerian markets was determined by Gas Chromatography- Mass Spectrometry. The capsaicinoids were extracted from the peppers using methanol as extractant and analyzed without need for derivatization. A total of eight (8) capsaicinoids were identified and quantitated: Capsaicin, Dihydrocapsaicin Dihydrocapsaicin 1, Dihydrocapsaicin 2, Norcapsaicin, Nordihydrocapsaicin 1, Nordihydrocapsaicin 2 and Nornordihydrocapsaicin though, not fully present in all the varieties. Dihydrocapsaicin 1, Dihydrocapsaicin 2, Nordihydrocapsaicin 1, Nordihydrocapsaicin 2 and Nornordihydrocapsaicin are isomers. Seven were identified in the Cameroun pepper variety, six in “Zaria atarugu” and Miango, and five in each of the remaining varieties. In all the peppers analyzed, capsaicin had the highest relative concentration, which ranged from 27.3% in the Cameroun variety to 49.38% in the “Zaria atarugu” variety. The sum of the relative concentrations of capsaicin and dihydrocapsaicin ranged from 47.03% in the “Miango” variety to 87.3% in the “Zaria atarugu” variety. Keywords: Capsaicinoids, Gas Chromatography-Mass Spectrometry, Methanol, Pepper ____________________________________________________________________________________________ INTRODUCTION Peppers are widely used in many parts of the world as a result of their valued sensory attributes; colour, purgency and aroma. -
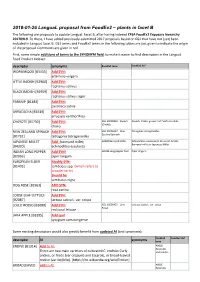
2018-01-26 Langual Proposal from Foodex2 – Plants in Facet B
2018-01-26 LanguaL proposal from FoodEx2 – plants in facet B The following are proposals to update LanguaL Facet B, after having indexed EFSA FoodEx2 Exposure hierarchy 20170919. To these, I have added previously-submitted 2017 proposals based on GS1 that have not (yet) been included in LanguaL facet B. GS1 terms and FoodEx2 terms in the following tables are just given to indicate the origin of the proposal. Comments are given in red. First, some simple additions of terms to the SYNONYM field, to make it easier to find descriptors in the LanguaL Food Product Indexer: descriptor synonyms FoodEx2 term FoodEx2 def WORMWOOD [B3433] Add SYN: artemisia vulgaris LITTLE RADISH [B2960] Add SYN: raphanus sativus BLACK RADISH [B2959] Add SYN: raphanus sativus niger PARSNIP [B1483] Add SYN: pastinaca sativa ARRACACHA [B3439] Add SYN: arracacia xanthorrhiza CHAYOTE [B1730] Add SYN: GS1 10006356 - Squash Squash, Choko, grown from Sechium edule (Choko) choko NEW ZEALAND SPINACH Add SYN: GS1 10006427 - New- Tetragonia tetragonoides Zealand Spinach [B1732] tetragonia tetragonoides JAPANESE MILLET Add : barnyard millet; A000Z Barnyard millet Echinochloa esculenta (A. Braun) H. Scholz, Barnyard millet or Japanese Millet. [B4320] echinochloa esculenta INDIAN LONG PEPPER Add SYN! A019B Long pepper fruit Piper longum [B2956] piper longum EUROPEAN ELDER Modify SYN: [B1403] sambucus spp. (which refers to broader term) Should be sambucus nigra DOG ROSE [B2961] ADD SYN: rosa canina LOOSE LEAF LETTUCE Add SYN: [B2087] lactusa sativa L. var. crispa LOLLO ROSSO [B2088] Add SYN: GS1 10006425 - Lollo Lactuca sativa L. var. crispa Rosso red coral lettuce JAVA APPLE [B3395] Add syn! syzygium samarangense Some existing descriptors would also greatly benefit from updated AI (and synonyms): FoodEx2 FoodEx2 def descriptor AI synonyms term ENDIVE [B1314] Add to AI: A00LD Escaroles There are two main varieties of cultivated C. -

Reimer Seeds Catalog
LCTRONICLCTRONIC CATALOGCATALOG Drying Hot Peppers HP320‐20 ‐ Achar Hot Peppers HP321‐10 ‐ Aci Sivri Hot Peppers 85 days. Capsicum annuum. Open 85 days. Capsicum annuum. Open Pollinated. The plant produces good yields Pollinated. The plant produces good yields of 3 ¼" long by 1" wide hot peppers. Peppers of 7 ½" long by ½" wide Cayenne type hot are hot, have medium thin flesh, and turn peppers. Peppers are medium hot, have from green to deep red when mature. The medium thin flesh, and turn from light plant has green stems, green leaves, and yellowish‐green to red when mature. The white flowers. Excellent for pickling and plant has green stems, green leaves, and seasoning spice. A variety from India. United white flowers. Excellent drying, pickling, and States Department of Agriculture, PI 640826. seasoning powder. An heirloom variety from Scoville Heat Units: 27,267. Turkey. HP21‐10 ‐ Afghan Hot Peppers HP358‐10 ‐ African Fish Hot Peppers 85 days. Capsicum annuum. Open 85 days. Capsicum annuum. Open Pollinated. The plant produces good yields Pollinated. The plant produces good yields of 3" long by ½" wide Cayenne hot peppers. of 1 ½" long by ½" wide hot peppers. Peppers are very hot, have medium thin Peppers are medium‐hot, have medium thin flesh, and turn from green to red when flesh, and turn from cream white with green mature. The plant has green stems, green stripes, to orange with brown stripes, then leaves, and white flowers. Excellent for to red when mature. The plant has Oriental cuisine and for making hot pepper variegated leaves. An African‐American flakes and seasoning spice powder. -

Cyclic Voltammetric Determination of Capsaicin by Using Electrochemically Deposited Tin and Reduced Graphene Oxide on Screen-Printed Carbon Electrodes
R ESEARCH ARTICLE doi: 10.2306/scienceasia1513-1874.2020.076 Cyclic voltammetric determination of capsaicin by using electrochemically deposited tin and reduced graphene oxide on screen-printed carbon electrodes Wasukamol Numphud, Orapin Chienthavorn, Wilai Siriwatcharapiboon∗ Department of Chemistry and the Center of Excellence for Innovation in Chemistry, Faculty of Science, Kasetsart University, Bangkok 10903 Thailand ∗Corresponding author, e-mail: [email protected] Received 28 May 2020 Accepted 19 Aug 2020 ABSTRACT: Tin and reduced graphene oxide (Sn/rGO) was prepared by an electrochemical deposition and modified on a screen-printed carbon electrode (SPCE) in order to improve the electrode selectivity and sensitivity for determination of capsaicin in real samples. The modified electrode was characterized for its surface morphology by scanning electron microscopy (SEM). An elemental analysis of prepared catalysts was confirmed by energy dispersive spectroscopy (EDS) and x-ray photoelectron spectroscopy (XPS). The experimental conditions influencing determination of capsaicin were optimized. The experiment was carried out in a sodium acetate buffer solution pH 3.0 at a scan rate of 50 mV/s. The Sn/rGO/SPCE showed a linear working range of 0.2–22 µM of capsaicin concentrations. The limit of detection and limit of quantification were 0.005 µM (S/N=3) and 0.02 µM (S/N=10), respectively. The prepared electrode was successfully applied to determine capsaicin in real chili samples and chili sauces. KEYWORDS: capsaicin detection, modified electrode, electrocatalyst, electrochemical sensor INTRODUCTION chromatography (HPLC) [6], gas chromatography- mass spectrometry (GC-MS) 15 , and liquid Chili is one of the most popular ingredients in food [ ] chromatography-mass spectrometry (LC-MS) 16 , owing to its unique characteristics for desirable [ ] have been reported. -

Note: the Letters 'F' and 'T' Following the Locators Refers to Figures and Tables
Index Note: The letters ‘f’ and ‘t’ following the locators refers to figures and tables cited in the text. A Acyl-lipid desaturas, 455 AA, see Arachidonic acid (AA) Adenophostin A, 71, 72t aa, see Amino acid (aa) Adenosine 5-diphosphoribose, 65, 789 AACOCF3, see Arachidonyl trifluoromethyl Adlea, 651 ketone (AACOCF3) ADP, 4t, 10, 155, 597, 598f, 599, 602, 669, α1A-adrenoceptor antagonist prazosin, 711t, 814–815, 890 553 ADPKD, see Autosomal dominant polycystic aa 723–928 fragment, 19 kidney disease (ADPKD) aa 839–873 fragment, 17, 19 ADPKD-causing mutations Aβ, see Amyloid β-peptide (Aβ) PKD1 ABC protein, see ATP-binding cassette protein L4224P, 17 (ABC transporter) R4227X, 17 Abeele, F. V., 715 TRPP2 Abbott Laboratories, 645 E837X, 17 ACA, see N-(p-amylcinnamoyl)anthranilic R742X, 17 acid (ACA) R807X, 17 Acetaldehyde, 68t, 69 R872X, 17 Acetic acid-induced nociceptive response, ADPR, see ADP-ribose (ADPR) 50 ADP-ribose (ADPR), 99, 112–113, 113f, Acetylcholine-secreting sympathetic neuron, 380–382, 464, 534–536, 535f, 179 537f, 538, 711t, 712–713, Acetylsalicylic acid, 49t, 55 717, 770, 784, 789, 816–820, Acrolein, 67t, 69, 867, 971–972 885 Acrosome reaction, 125, 130, 301, 325, β-Adrenergic agonists, 740 578, 881–882, 885, 888–889, α2 Adrenoreceptor, 49t, 55, 188 891–895 Adult polycystic kidney disease (ADPKD), Actinopterigy, 223 1023 Activation gate, 485–486 Aframomum daniellii (aframodial), 46t, 52 Leu681, amino acid residue, 485–486 Aframomum melegueta (Melegueta pepper), Tyr671, ion pathway, 486 45t, 51, 70 Acute myeloid leukaemia and myelodysplastic Agelenopsis aperta (American funnel web syndrome (AML/MDS), 949 spider), 48t, 54 Acylated phloroglucinol hyperforin, 71 Agonist-dependent vasorelaxation, 378 Acylation, 96 Ahern, G. -

TRP CHANNELS AS THERAPEUTIC TARGETS TRP CHANNELS AS THERAPEUTIC TARGETS from Basic Science to Clinical Use
TRP CHANNELS AS THERAPEUTIC TARGETS TRP CHANNELS AS THERAPEUTIC TARGETS From Basic Science to Clinical Use Edited by ARPAD SZALLASI MD, PHD Department of Pathology, Monmouth Medical Center, Long Branch, NJ, USA AMSTERDAM • BOSTON • HEIDELBERG • LONDON NEW YORK • OXFORD • PARIS • SAN DIEGO SAN FRANCISCO • SINGAPORE • SYDNEY • TOKYO Academic Press is an imprint of Elsevier Academic Press is an imprint of Elsevier 125 London Wall, London, EC2Y 5AS, UK 525 B Street, Suite 1800, San Diego, CA 92101-4495, USA 225 Wyman Street, Waltham, MA 02451, USA The Boulevard, Langford Lane, Kidlington, Oxford OX5 1GB, UK First published 2015 Copyright © 2015 Elsevier Inc. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. Details on how to seek permission, further information about the Publisher’s permissions policies and our arrangement with organizations such as the Copyright Clearance Center and the Copyright Licensing Agency, can be found at our website: www.elsevier.com/permissions This book and the individual contributions contained in it are protected under copyright by the Publisher (other than as may be noted herein). Notices Knowledge and best practice in this field are constantly changing. As new research and experience broaden our understanding, changes in research methods, professional practices, or medical treatment may become necessary. Practitioners and researchers must always rely on their own experience and knowledge in evaluating and using any information, methods, compounds, or experiments described herein. -

Capsicum Annum L.) in Southern USA
ACTA AGRÍCOLA Y PECUARIA 6: E0061006 SCIENTIFIC ARTICLE https://doi.org/10.30973/aap/2020.6.0061006 (April 27, 2020) Effect of water stress on functional and marketable properties of roasted Big Jim chili pepper (Capsicum annum L.) in Southern USA Efecto del estrés hídrico en las propiedades funcionales y comerciales del chile rostizado Big Jim (Capsicum annum L.) en el sur de Estados Unidos Nancy Flores¹, Efrén Delgado¹, Stephanie Walker¹, Juan Rojas-Contreras², Gerardo Pámanes-Carrasco³* 1College of Agricultural, Consumer and Environmental Sciences, New Mexico State University, 1780 E University Ave, 88003, Las Cruces, New Mexico, United States of America. 2Instituto Tecnológico de Durango, Tecnológico Nacional de México, Boulevard Felipe Pescador #1830 Oriente, 34080, Durango, Durango, México. 3CONACYT-UJED, Instituto de Silvivultura e Industria de la Madera, Universidad Juárez del Estado de Durango, Boulevard Guadiana #501, Ciudad Universitaria, 34120, Durango, Durango, México. *Corresponding author: [email protected] Reception date: abstract January 19, 2020 This study aimed to evaluate the effect of water stress on spiciness, fatty acids, and aroma compound profile of roasted Big Jim chili. A flooded furrow irrigation system for chili Acceptance date: peppers production was utilized with 4 irrigation treatments: every 7, 9, 11, and 13 days March 18, 2020 for W1, W2, W3, and W4, respectively, in a completely randomized block design. Capsai- Online publication date: cinoid content was increased (~160%) by increasing water stress (P<0.05). However, the April 27, 2020 roasting process reduced the capsaicinoids content (P<0.05). Contents of linoleic, palmitic, and arachidonic acids were not affected. Water stress reduced hexanal and linalool content by approximately 64 and 72%, respectively (P<0.05), whereas 2-isobutyl-3-methoxypyrazi- ne content increased (P<0.05). -

Biomolecules
biomolecules Article Inhibitory Activity of Flavonoids, Chrysoeriol and Luteolin-7-O-Glucopyranoside, on Soluble Epoxide Hydrolase from Capsicum chinense Jang Hoon Kim and Chang Hyun Jin * Advanced Radiation Technology Institute, Korea Atomic Energy Research Institute, Jeongeup, Jeollabuk-do 56212, Korea; [email protected] * Correspondence: [email protected]; Tel.: +82-63-570-3162; Fax: +82-63-570-3159 Received: 5 December 2019; Accepted: 22 January 2020; Published: 24 January 2020 Abstract: Three flavonoids derived from the leaves of Capsicum chinense Jacq. were identified as chrysoeriol (1), luteolin-7-O-glucopyranoside (2), and isorhamnetin-7-O-glucopyranoside (3). They had IC values of 11.6 2.9, 14.4 1.5, and 42.7 3.5 µg/mL against soluble epoxide hydrolase 50 ± ± ± (sEH), respectively. The three inhibitors (1–3) were found to non-competitively bind into the allosteric site of the enzyme with K values of 10.5 3.2, 11.9 2.8 and 38.0 4.1 µg/mL, respectively. The i ± ± ± potential inhibitors 1 and 2 were located at the left edge ofa U-tube shape that contained the enzyme active site. Additionally, we observed changes in several factors involved in the binding of these complexes under 300 K and 1 bar. Finally, it was confirmed that each inhibitor, 1 and 2, could be complexed with sEH by the “induced fit” and “lock-and-key” models. Keywords: flavonoids; soluble epoxide hydrolase; non-competitive mode; induced fit; lock-and-key 1. Introduction Arachidonic acid is converted to epoxyeicosatrienoic acids (EETs) by cytochrome P450 epoxygenase [1]. EETs exist as four regioisomeric metabolites; 5,6-, 8,9-, 11,12- and 14,15-EETs [1].