A New Species of Eriophyoid Mite, Aceria Tripuraensis Sp
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

15. HIBISCUS Linnaeus, Sp. Pl. 2: 693. 1753, Nom. Cons
Flora of China 12: 286–294. 2007. 15. HIBISCUS Linnaeus, Sp. Pl. 2: 693. 1753, nom. cons. 木槿属 mu jin shu Bombycidendron Zollinger & Moritzi; Fioria Mattei; Furcaria (Candolle) Kosteletzky (1836), not Desvaux (1827); Hibiscus sect. Furcaria Candolle; H. sect. Sabdariffa Candolle; Ketmia Miller; Sabdariffa (Candolle) Kosteletzky; Solandra Murray (1785), not Linnaeus (1759), nor Swartz (1787), nom. cons.; Talipariti Fryxell. Shrubs, subshrubs, trees, or herbs. Leaf blade palmately lobed or entire, basal veins 3 or more. Flowers axillary, usually solitary, sometimes subterminal and ± congested into a terminal raceme, 5-merous, bisexual. Epicalyx lobes 5 to many, free or connate at base, rarely very short (H. schizopetalus) or absent (H. lobatus). Calyx campanulate, rarely shallowly cup-shaped or tubular, 5-lobed or 5-dentate, persistent. Corolla usually large and showy, variously colored, often with dark center; petals adnate at base to staminal tube. Filament tube well developed, apex truncate or 5-dentate; anthers throughout or only on upper half of tube. Ovary 5-loculed or, as a result of false partitions, 10-loculed; ovules 3 to many per locule; style branches 5; stigmas capitate. Fruit a capsule, cylindrical to globose, valves 5, dehiscence loculicidal and sometimes partially septicidal or indehiscent (H. vitifolius Linnaeus). Seeds reniform, hairy or glandular verrucose. About 200 species: tropical and subtropical regions; 25 species (12 endemic, four introduced) in China. According to recent molecular studies (Pfeil et al., Syst. Bot. 27: 333–350. 2002), Hibiscus is paraphyletic, and as more taxa are sampled and a more robust phylogeny is constructed, the genus undoubtedly will be recast. Species of other genera of Hibisceae found in China, such as Abelmoschus, Malvaviscus, and Urena, fall within a monophyletic Hibiscus clade. -

Technical Guidelines for Reforestation at Ex-Coal-Mining Areas
Technical Guidelines for Reforestation at Ex-Coal-Mining Areas - Based on the outcomes of experimental reforestation activities at ex-coal-mining areas in South Kalimantan, Indonesia - Japan International Forestry Promotion and Cooperation Center (JIFPRO) March 2015 Technical Guidelines for Reforestation at Ex-Coal-Mining Areas - Based on the outcomes of experimental reforestation activities at ex-coal-mining areas in South Kalimantan, Indonesia - Eiichiro Nakama, Seiichi Ohta, Yasuo Ohsumi, Tokunori Mori and Satohiko Sasaki Japan International Forestry Promotion and Cooperation Center Fakhrur Razie, Hamdani Fauzi and Mahrus Aryadi Lambung Mangkurat University, Indonesia Japan International Forestry Promotion and Cooperation Center March 2015 Foreword During the past decades, deforestation and forest degradation continues especially in developing countries. According to the report of the Food and Agriculture Organization of the United Nation (FAO), approximately 13 million hectors of global forests have been lost annually due to forest land conversion to other land uses, forest fires and natural disasters, while reforestation and natural regeneration account for an increase of approx. 7.8 million hectors of forest cover. This means the net loss of global forest is estimated at 5.2 million hectors. Adverse impacts of forest conversion to farmland can be minimized as far as the land is properly used and managed in a sustainable manner. However, in some cases, problem soils are exposed and abandoned as degraded land. Deforestation by mining is a big issue these years. Problem soils such as strong acid soils and/or too much heavy metal soils appear at the ex-mining areas. In some cases it is too difficult to reforestate. -

Origin and Diversification of Hibiscus Glaber 1061
Molecular Ecology (2005) 14, 1059–1071 doi: 10.1111/j.1365-294X.2005.02462.x OriginBlackwell Publishing, Ltd. and diversification of Hibiscus glaber, species endemic to the oceanic Bonin Islands, revealed by chloroplast DNA polymorphism KOJI TAKAYAMA,* TETSUO OHI-TOMA,* HIROSHI KUDOH† and HIDETOSHI KATO‡ *Botanical Gardens, Graduate School of Science, The University of Tokyo, Hakusan 3-7-1, Bunkyo-ku, Tokyo 112–0001, Japan, †Department of Biology, Faculty of Science, Kobe University, Nada-ku, Kobe 657–8501, Japan, ‡Makino Herbarium, Graduate School of Science, Tokyo Metropolitan University, Minami-osawa 1–1, Hachioji, Tokyo 192–0397, Japan Abstract Two woody Hibiscus species co-occur in the Bonin Islands of the northwestern Pacific Ocean: Hibiscus glaber Matsum. is endemic to the islands, and its putative ancestral species, Hibiscus tiliaceus L., is widely distributed in coastal areas of the tropics and subtropics. To infer isolating mechanisms that led to speciation of H. glaber and the processes that resulted in co-occurrence of the two closely related species on the Bonin Islands, we conducted molecular phylogenetic analyses on chloroplast DNA (cpDNA) sequences. Materials collected from a wide area of the Pacific and Indian Oceans were used, and two closely related species, Hibiscus hamabo Siebold Zucc. and Hibiscus macrophyllus Roxb., were also included in the analyses. The constructed tree suggested that H. glaber has been derived from H. tiliaceus, and that most of the modern Bonin populations of H. tiliaceus did not share most recent ancestry with H. glaber. Geographic isolation appears to be the most important mechanism in the speciation of H. glaber. -

Mangrove Guidebook for Southeast Asia
RAP PUBLICATION 2006/07 MANGROVE GUIDEBOOK FOR SOUTHEAST ASIA The designations and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its frontiers or boundaries. The opinions expressed in this publication are those of the authors alone and do not imply any opinion whatsoever on the part of FAO. Authored by: Wim Giesen, Stephan Wulffraat, Max Zieren and Liesbeth Scholten ISBN: 974-7946-85-8 FAO and Wetlands International, 2006 Printed by: Dharmasarn Co., Ltd. First print: July 2007 For copies write to: Forest Resources Officer FAO Regional Office for Asia and the Pacific Maliwan Mansion Phra Atit Road, Bangkok 10200 Thailand E-mail: [email protected] ii FOREWORDS Large extents of the coastlines of Southeast Asian countries were once covered by thick mangrove forests. In the past few decades, however, these mangrove forests have been largely degraded and destroyed during the process of development. The negative environmental and socio-economic impacts on mangrove ecosystems have led many government and non- government agencies, together with civil societies, to launch mangrove conservation and rehabilitation programmes, especially during the 1990s. In the course of such activities, programme staff have faced continual difficulties in identifying plant species growing in the field. Despite a wide availability of mangrove guidebooks in Southeast Asia, none of these sufficiently cover species that, though often associated with mangroves, are not confined to this habitat. -

Threatened Ecosystems of Myanmar
Threatened ecosystems of Myanmar An IUCN Red List of Ecosystems Assessment Nicholas J. Murray, David A. Keith, Robert Tizard, Adam Duncan, Win Thuya Htut, Nyan Hlaing, Aung Htat Oo, Kyaw Zay Ya and Hedley Grantham 2020 | Version 1.0 Threatened Ecosystems of Myanmar. An IUCN Red List of Ecosystems Assessment. Version 1.0. Murray, N.J., Keith, D.A., Tizard, R., Duncan, A., Htut, W.T., Hlaing, N., Oo, A.H., Ya, K.Z., Grantham, H. License This document is an open access publication licensed under a Creative Commons Attribution-Non- commercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0). Authors: Nicholas J. Murray University of New South Wales and James Cook University, Australia David A. Keith University of New South Wales, Australia Robert Tizard Wildlife Conservation Society, Myanmar Adam Duncan Wildlife Conservation Society, Canada Nyan Hlaing Wildlife Conservation Society, Myanmar Win Thuya Htut Wildlife Conservation Society, Myanmar Aung Htat Oo Wildlife Conservation Society, Myanmar Kyaw Zay Ya Wildlife Conservation Society, Myanmar Hedley Grantham Wildlife Conservation Society, Australia Citation: Murray, N.J., Keith, D.A., Tizard, R., Duncan, A., Htut, W.T., Hlaing, N., Oo, A.H., Ya, K.Z., Grantham, H. (2020) Threatened Ecosystems of Myanmar. An IUCN Red List of Ecosystems Assessment. Version 1.0. Wildlife Conservation Society. ISBN: 978-0-9903852-5-7 DOI 10.19121/2019.Report.37457 ISBN 978-0-9903852-5-7 Cover photos: © Nicholas J. Murray, Hedley Grantham, Robert Tizard Numerous experts from around the world participated in the development of the IUCN Red List of Ecosystems of Myanmar. The complete list of contributors is located in Appendix 1. -
The Risk Assessment
Designation = Evaluate WRA Score = 3 Family: Malvaceae Taxon: Talipariti macrophyllum Synonym: Hibiscus macrophyllus Roxb. ex Hornem. (bas Common Name: Largeleaf Rosemallow Pariti macrophyllum (Roxburgh ex Horneman Questionaire : current 20090513 Assessor: Chuck Chimera Designation: EVALUATE Status: Assessor Approved Data Entry Person: Chuck Chimera WRA Score 3 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? y=1, n=-1 103 Does the species have weedy races? y=1, n=-1 201 Species suited to tropical or subtropical climate(s) - If island is primarily wet habitat, then (0-low; 1-intermediate; 2- High substitute "wet tropical" for "tropical or subtropical" high) (See Appendix 2) 202 Quality of climate match data (0-low; 1-intermediate; 2- High high) (See Appendix 2) 203 Broad climate suitability (environmental versatility) y=1, n=0 204 Native or naturalized in regions with tropical or subtropical climates y=1, n=0 y 205 Does the species have a history of repeated introductions outside its natural range? y=-2, ?=-1, n=0 ? 301 Naturalized beyond native range y = 1*multiplier (see y Appendix 2), n= question 205 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see n Appendix 2) 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see n Appendix 2) 304 Environmental weed n=0, y = 2*multiplier (see n Appendix 2) 305 Congeneric weed n=0, y = 1*multiplier (see y Appendix 2) 401 Produces spines, thorns or burrs y=1, n=0 n 402 Allelopathic y=1, n=0 n 403 Parasitic y=1, n=0 -
Vegetation Physiognomy, Diversity, Evenness and Abundance of Trees in the Protected Sirimau Forest Soya Village, Ambon City
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by International Institute for Science, Technology and Education (IISTE): E-Journals Journal of Natural Sciences Research www.iiste.org ISSN 2224-3186 (Paper) ISSN 2225-0921 (Online) Vol.6, No.8, 2016 Vegetation Physiognomy, Diversity, Evenness and Abundance of Trees in the Protected Sirimau Forest Soya Village, Ambon City Pamella Mercy Papilaya Program Studi Pendidikan Biologi FKIP Unpatti Abstract Sirimau protected forest is one forest area that still has the characteristics of the forest consists of primary forest and old secondary forest. Particularly for the purpose of education and research, Sirimau Protected Forest Area has a diversity of flora and fauna as well as the high potential tourist attraction. This study aims to determine the condition of the vegetation Physiognomy, evenness, the abundance and diversity of tree species in the Forest Preserve Sirimau Ambon City. In general, Ambon city Sirimau Protected forests consist of protected forest vegetation characteristic of the region indicate that the tropical rainforest is typically display the five strata or layers of the tree. The number of species found in protected forests Sirimau Ambon city a total of 93 species. Gandaria (Bouea macrophylla ) has the highest frequency of protected forest in Sirimau, followed by Durio zibethinus and Lansium domesticum . The results of the analysis of diversity, evenness and abundance on all observation stations index values obtained are relatively the same. Measurement of Importance Value Index (IVI) do indicate a shift in the dominance of plant species and the difference between the research stations throughout the observation station. -
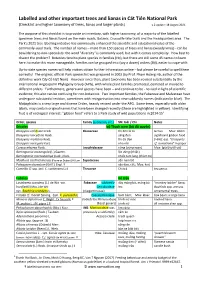
Labelled and Other Important Trees and Lianas in Cát Tiên National Park (Checklist and Higher Taxonomy of Trees, Lianas and Larger Plants) V.2 Update: 18 August 2021
Labelled and other important trees and lianas in Cát Tiên National Park (Checklist and higher taxonomy of trees, lianas and larger plants) v.2 update: 18 August 2021 The purpose of this checklist is to provide an inventory, with higher taxonomy, of a majority of the labelled specimen trees and lianas found on the main roads, Botanic, Crocodile-lake trails and the Headquarters area. The Park's 2021 tree labelling initiative has enormously enhanced the scientific and educational value of the commonly-used trails. The number of names – more than 150 species of trees and lianas (woody vines) - can be bewildering to non-specialists: the word "diversity" is commonly used, but with it comes complexity. How best to dissect the problem? Botanists tend to place species in families (Họ), but there are still some 45 names to learn here; to make this more manageable, families can be grouped into (say a dozen) orders (Bộ), easier to cope-with. Up-to-date species names will help visitors obtain further information online – but please be careful to spell them correctly! The original, official Park species list was prepared in 2002 (by Prof. Phạm Hoàng Hộ, author of the definitive work Cây Cỏ Việt Nam). However since then, plant taxonomy has been revised substantially by the international Angiosperm Phylogeny Group (APG), with whole plant families promoted, demoted or moved to different orders. Furthermore, genera and species have been – and continue to be - revised in light of scientific evidence; this also can be confusing for non-botanists. Two important families, the Fabaceae and Malvaceae have undergone substantial revision, sometimes with reorganisation into new subfamily names (indicated in blue). -

MALVACEAE 锦葵科 Jin Kui Ke Tang Ya (唐亚)1; Michael G
MALVACEAE 锦葵科 jin kui ke Tang Ya (唐亚)1; Michael G. Gilbert2, Laurence J. Dorr3 Herbs, shrubs, or less often trees; indumentum usually with peltate scales or stellate hairs. Leaves alternate, stipulate, petiolate; leaf blade usually palmately veined, entire or various lobed. Flowers solitary, less often in small cymes or clusters, axillary or subterminal, often aggregated into terminal racemes or panicles, usually conspicuous, actinomorphic, usually bisexual (unisexual in Kydia). Epicalyx often present, forming an involucre around calyx, 3- to many lobed. Sepals 5, valvate, free or connate. Petals 5, free, contorted, or imbricate, basally adnate to base of filament tube. Stamens usually very many, filaments connate into tube; anthers 1-celled. Pollen spiny. Ovary superior, with 2–25 carpels, often separating from one another and from axis; ovules 1 to many per locule; style as many or 2 × as many as pistils, apex branched or capitate. Fruit a loculicidal capsule or a schizocarp, separating into individual mericarps, rarely berrylike when mature (Malvaviscus); carpels sometimes with an endoglossum (a crosswise projection from back wall of carpel to make it almost completely septate). Seeds often reniform, glabrous or hairy, sometimes conspicuously so. About 100 genera and ca. 1000 species: tropical and temperate regions of N and S Hemisphere; 19 genera (four introduced) and 81 species (24 endemic, 16 introduced) in China. Molecular studies have shown that the members of the Bombacaceae, Malvaceae, Sterculiaceae, and Tiliaceae form a very well-defined mono- phyletic group that is divided into ten also rather well-defined clades, only two of which correspond to the traditional families Bombacaceae and Mal- vaceae. -

The Traditional Uses, Phytochemistry and Pharmacology of Genus Hibiscus: a Review
European Journal of Medicinal Plants 32(4): 1-37, 2021; Article no.EJMP.68419 ISSN: 2231-0894, NLM ID: 101583475 The Traditional Uses, Phytochemistry and Pharmacology of Genus Hibiscus: A Review Manish Kapoor1*, Gurdeep Kaur1*, Navneet Kaur1, Chanchal Sharma1, Kajal Batra1 and Davinderpal Singh1 1Department of Botany, Punjabi University, Patiala, India. Authors’ contributions This work was carried out in collaboration among all authors. Authors MK and DS designed the study and analysed. Authors GK, NK, CS and KB interpreted and prepared the manuscript. All authors read and approved the final manuscript. Article Information DOI: 10.9734/EJMP/2021/v32i430382 Editor(s): (1) Dr. Daniela Rigano, University Federico II of Naples, Italy. (2) Prof. Marcello Iriti, Milan State University, Italy. Reviewers: (1) Aruna Bhushan, Belagavi Institute of Medical Sciences, India. (2) Dzulsuhaimi Daud, Universiti Teknologi MARA, Malaysia. (3) Perumal Pandurangan, Dr. MGR Medical University, India. Complete Peer review History: http://www.sdiarticle4.com/review-history/68419 Received 14 March 2021 Accepted 24 May 2021 Review Article Published 03 June 2021 ABSTRACT The genus Hibiscus belongs to the mallow family, Malvaceae comprising of about 275 species growing in tropical and sub tropical areas. The various species of genus Hibiscus have been used as traditional medicine all over the world. There are numerous reports of their traditional medicinal uses in various countries like India, Nigeria, China, and Srilanka etc. to cure various ailments such as hypertension, cardiac diseases, stomach-ache, urine problems, skin diseases and many more. Based on the historical knowledge, various pharmacological and phytochemical studies on some species of the genus Hibiscus have been done. -

Biogeography and Phylogenetics of the Hawaiian Endemic Hibiscadelphus, Hau Kuahiwi
BIOGEOGRAPHY AND PHYLOGENETICS OF THE HAWAIIAN ENDEMIC HIBISCADELPHUS, HAU KUAHIWI (MALVACEAE) A THESIS SUBMITTED TO THE GRADUATE DIVISION OF THE UNIVERSITY OF HAWAI’I IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE MASTER OF SCIENCE IN BOTANY (ECOLOGY, EVOLUTION, AND CONSERVATION BIOLOGY) MAY 2020 By Solomon J. Champion THESIS COMMITTEE: Clifford Morden, Chair Alison Sherwood Matthew Knope Acknowledgements I would like to thank all of my professors and especially my thesis committee members; Dr. Clifford Morden, Dr. Alison Sherwood, and Dr. Matthew Knope. I greatly appreciate the help of our lab manager, Mitsuko Yorkston, for all her help in the lab and with sequence analysis. Mahalo nui to David Orr at the Waimea Valley Botanical Gardens. I am also very grateful to Liz Huppman for her help in sharing her Hibiscus expertise. I am very thankful for the assistance of Barbara Kennedy at the Bishop Museum. I would like to acknowledge the organizations dedicated to protecting Hawaii’s rare and endangered plants: Hawaii Volcanoes National Park, USGS, and The National Tropical Botanical Garden. I would like to also thank Hank Oppenheimer for all his help with this project, Dr. Sterling Keeley for her encouragement, and Dr. Jonathan Price, Dr. Rebecca Chong, and Dr. Michael Kantar for their assistance. Specifically, I would like to thank Dr. Daniel Rubinoff for his Systematics and Phylogenetics course and the Teaching Assistants, Dr. Camiel Doorenweerd and Dr. Michael San Jose. This work is one such that is built upon much of the previous efforts of others, both conceptually and academically. I am specifically referring to the Progression Rule which, while initially described by Willi Hennig in his book “Phylogenetic Systematics” in 1966, was later reexamined and thoughtfully applied to many lineages of the Hawaiian flora and fauna in the book “ Hawaiian Biogeography: Evolution on a Hot Spot Archipelago”, an excellent publication from the Smithsonian edited by Vicki A. -

Molecular Systematics of Abelmoschus (Malvaceae) And
Molecular systematics of Abelmoschus (Malvaceae) and genetic diversity within the cultivated species of this genus based on nuclear ITS and chloroplast rpL16 sequence data Olaf Werner, Mahmoud Magdy & Rosa María Ros Genetic Resources and Crop Evolution An International Journal ISSN 0925-9864 Volume 63 Number 3 Genet Resour Crop Evol (2016) 63:429-445 DOI 10.1007/s10722-015-0259-x 1 23 Your article is protected by copyright and all rights are held exclusively by Springer Science +Business Media Dordrecht. This e-offprint is for personal use only and shall not be self- archived in electronic repositories. If you wish to self-archive your article, please use the accepted manuscript version for posting on your own website. You may further deposit the accepted manuscript version in any repository, provided it is only made publicly available 12 months after official publication or later and provided acknowledgement is given to the original source of publication and a link is inserted to the published article on Springer's website. The link must be accompanied by the following text: "The final publication is available at link.springer.com”. 1 23 Author's personal copy Genet Resour Crop Evol (2016) 63:429–445 DOI 10.1007/s10722-015-0259-x RESEARCH ARTICLE Molecular systematics of Abelmoschus (Malvaceae) and genetic diversity within the cultivated species of this genus based on nuclear ITS and chloroplast rpL16 sequence data Olaf Werner . Mahmoud Magdy . Rosa Marı´a Ros Received: 7 January 2015 / Accepted: 27 April 2015 / Published online: 9 May 2015 Ó Springer Science+Business Media Dordrecht 2015 Abstract The genus Abelmoschus includes several species.