Caspian Tern (Sterna Caspia)
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Order CHARADRIIFORMES: Waders, Gulls and Terns Suborder LARI
Text extracted from Gill B.J.; Bell, B.D.; Chambers, G.K.; Medway, D.G.; Palma, R.L.; Scofield, R.P.; Tennyson, A.J.D.; Worthy, T.H. 2010. Checklist of the birds of New Zealand, Norfolk and Macquarie Islands, and the Ross Dependency, Antarctica. 4th edition. Wellington, Te Papa Press and Ornithological Society of New Zealand. Pages 191, 223 & 227-228. Order CHARADRIIFORMES: Waders, Gulls and Terns The family sequence of Christidis & Boles (1994), who adopted that of Sibley et al. (1988) and Sibley & Monroe (1990), is followed here. Suborder LARI: Skuas, Gulls, Terns and Skimmers Condon (1975) and Checklist Committee (1990) recognised three subfamilies within the Laridae (Larinae, Sterninae and Megalopterinae) but this division has not been widely adopted. We follow Gochfeld & Burger (1996) in recognising gulls in one family (Laridae) and terns and noddies in another (Sternidae). The sequence of species for Stercorariidae and Laridae follows Peters (1934) and for Sternidae follows Bridge et al. (2005). Family LARIDAE Rafinesque: Gulls Laridia Rafinesque, 1815: Analyse de la Nature: 72 – Type genus Larus Linnaeus, 1758. Genus Larus Linnaeus Larus Linnaeus, 1758: Syst. Nat., 10th edition 1: 136 – Type species (by subsequent designation) Larus marinus Linnaeus. Gavia Boie, 1822: Isis von Oken, Heft 10: col. 563 – Type species (by subsequent designation) Larus ridibundus Linnaeus. Junior homonym of Gavia Moehring, 1758. Hydrocoleus Kaup, 1829: Skizz. Entw.-Gesch. Eur. Thierw.: 113 – Type species (by subsequent designation) Larus minutus Linnaeus. Chroicocephalus Eyton, 1836: Cat. Brit. Birds: 53 – Type species (by monotypy) Larus cucullatus Reichenbach = Larus pipixcan Wagler. Gelastes Bonaparte, 1853: Journ. für Ornith. -

Non-Living (Abiotic) Elements Shape Habitat
Forest Birds Fast Facts LIGHT: Dominated by tall trees, layered canopy = very shady, openings made from a fallen tree provide sunny areas AIR: Trees slow wind, except along forest edges and openings. Shade = cool temps WATER: Fog and rain collect in tree branches and drip to ground. SOIL: Lots of organics (needles, decaying leaves, tree trunks) makes lots of space for air and water. Soil absorbs and holds water like a sponge. Varied Thrush • Slender bill good at gleaning soft foods like insects, pillbugs, snails, worms, fruits and some seeds from ground. • Hops and pauses to look for food. Flips leaves and debris with bill. • Perching feet – three toes forward, hind toe back. • Can sing 2 separate notes at same time and breathe while singing. Common Raven Varied Thrush • Versatile beak. Eats everything from carrion and garbage, to eggs, nestlings, insects, seeds, rodents and fruit. • Strong, sturdy feet and grasping toes to manipulate food and perch. • Acrobatic flight, hops on ground. Uses sight to find food. Northern Flicker • The toes are placed two forward, two back to grip firmly, while the tail feathers are stiff and pointed to help brace while pounding. • Bill is shaped like a chisel. Flickers don’t excavate as much as other types of woodpeckers so have a slightly curved bill and less sharp. Common Raven • Flickers eat insects and are especially fond of ants (flickers will forage on the ground as well as on trees). Probe and explore crevices. • Distinctive flight – flap, dip, flap, dip • Woodpeckers have long, sticky and barbed tongues to extract bugs. -
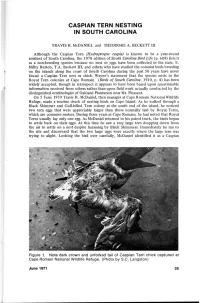
Caspian Tern Nesting in South Carolina
CASPIAN TERN NESTING IN SOUTH CAROLINA TRAVIS H. McDANIEL and THEODORE A. BECKETT III Although the Caspian Tern (Hydroprogne caspia) is known to be a year-round resident of South Carolina, the 1970 edition of South Carolina Bird Life (p. 608) lists it as a non-breeding species because no nest or eggs have been collected in the state. E. Milby Burton, T.A. Beckett III, and others who have studied the colonial birds breeding on the islands along the coast of South Carolina during the past 50 years have never found a Caspian Tern nest or chick. Wayne's statement that the species nests in the Royal Tern colonies at Cape Romain (Birds of South Carolina, 1910, p. 4) has been widely accepted, though in retrospect it appears to have been based upon questionable information received from others rather than upon field work actually conducted by the distinguished ornithologist of Oakland Plantation near Mt. Pleasant. On 5 June 1970 Travis H. McDaniel, then manager at Cape Romain National Wildlife Refuge, made a routine check of nesting birds on Cape Island. As he walked through a Black Skimmer and Gull-billed Tern colony at the south end of the island, he noticed two tern eggs that were appreciably larger than those normally laid by Royal Terns, which are common nesters. During three years at Cape Romain, he had noted that Royal Terns usually lay only one egg. As McDaniel returned to his patrol truck, the birds began to settle back on their eggs. At this time he saw a very large tern dropping down from the air to settle on a nest despite harassing by Black Skimmers. -

US Fish & Wildlife Service Seabird Conservation Plan—Pacific Region
U.S. Fish & Wildlife Service Seabird Conservation Plan Conservation Seabird Pacific Region U.S. Fish & Wildlife Service Seabird Conservation Plan—Pacific Region 120 0’0"E 140 0’0"E 160 0’0"E 180 0’0" 160 0’0"W 140 0’0"W 120 0’0"W 100 0’0"W RUSSIA CANADA 0’0"N 0’0"N 50 50 WA CHINA US Fish and Wildlife Service Pacific Region OR ID AN NV JAP CA H A 0’0"N I W 0’0"N 30 S A 30 N L I ort I Main Hawaiian Islands Commonwealth of the hwe A stern A (see inset below) Northern Mariana Islands Haw N aiian Isla D N nds S P a c i f i c Wake Atoll S ND ANA O c e a n LA RI IS Johnston Atoll MA Guam L I 0’0"N 0’0"N N 10 10 Kingman Reef E Palmyra Atoll I S 160 0’0"W 158 0’0"W 156 0’0"W L Howland Island Equator A M a i n H a w a i i a n I s l a n d s Baker Island Jarvis N P H O E N I X D IN D Island Kauai S 0’0"N ONE 0’0"N I S L A N D S 22 SI 22 A PAPUA NEW Niihau Oahu GUINEA Molokai Maui 0’0"S Lanai 0’0"S 10 AMERICAN P a c i f i c 10 Kahoolawe SAMOA O c e a n Hawaii 0’0"N 0’0"N 20 FIJI 20 AUSTRALIA 0 200 Miles 0 2,000 ES - OTS/FR Miles September 2003 160 0’0"W 158 0’0"W 156 0’0"W (800) 244-WILD http://www.fws.gov Information U.S. -

Broad Tapeworms (Diphyllobothriidae)
IJP: Parasites and Wildlife 9 (2019) 359–369 Contents lists available at ScienceDirect IJP: Parasites and Wildlife journal homepage: www.elsevier.com/locate/ijppaw Broad tapeworms (Diphyllobothriidae), parasites of wildlife and humans: T Recent progress and future challenges ∗ Tomáš Scholza, ,1, Roman Kuchtaa,1, Jan Brabeca,b a Institute of Parasitology, Biology Centre of the Czech Academy of Sciences, Branišovská 31, 370 05, České Budějovice, Czech Republic b Natural History Museum of Geneva, PO Box 6434, CH-1211, Geneva 6, Switzerland ABSTRACT Tapeworms of the family Diphyllobothriidae, commonly known as broad tapeworms, are predominantly large-bodied parasites of wildlife capable of infecting humans as their natural or accidental host. Diphyllobothriosis caused by adults of the genera Dibothriocephalus, Adenocephalus and Diphyllobothrium is usually not a life-threatening disease. Sparganosis, in contrast, is caused by larvae (plerocercoids) of species of Spirometra and can have serious health consequences, exceptionally leading to host's death in the case of generalised sparganosis caused by ‘Sparganum proliferum’. While most of the definitive wildlife hosts of broad tapeworms are recruited from marine and terrestrial mammal taxa (mainly carnivores and cetaceans), only a few diphyllobothriideans mature in fish-eating birds. In this review, we provide an overview the recent progress in our understanding of the diversity, phylogenetic relationships and distribution of broad tapeworms achieved over the last decade and outline the prospects of future research. The multigene family-wide phylogeny of the order published in 2017 allowed to propose an updated classi- fication of the group, including new generic assignment of the most important causative agents of human diphyllobothriosis, i.e., Dibothriocephalus latus and D. -

Characterization of Vertically and Cross-Species Transmitted Viruses in the Cestode Parasite 2 Schistocephalus Solidus
bioRxiv preprint doi: https://doi.org/10.1101/803247; this version posted October 13, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 1 Characterization of vertically and cross-species transmitted viruses in the cestode parasite 2 Schistocephalus solidus 3 Megan A Hahna, Karyna Rosariob, Pierrick Lucasc, Nolwenn M Dheilly a# 4 5 a School of Marine and Atmospheric Sciences, Stony Brook University, Stony Brook NY, USA 6 b College of Marine Science, University of South Florida, Saint Petersburg, FL, USA 7 c ANSES, Agence Nationale de Sécurité Sanitaire de l’Alimentation, de l’Environnement et du 8 Travail - Laboratoire de Ploufragan-Plouzané, Unité Génétique Virale de Biosécurité, 9 Ploufragan, France 10 11 # Address correspondence to Nolwenn M Dheilly: [email protected] 12 1 bioRxiv preprint doi: https://doi.org/10.1101/803247; this version posted October 13, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 13 Abstract 14 Parasitic flatworms (Neodermata) represent a public health and economic burden due to associated 15 debilitating diseases and limited therapeutic treatments available. Despite their importance, there 16 is scarce information regarding flatworm-associated microbes. We report the discovery of six RNA 17 viruses in the cestode Schistocephalus solidus. -

The Parasite Schistocephalus Solidus Secretes Proteins With
bioRxiv preprint doi: https://doi.org/10.1101/2020.02.03.932509; this version posted July 16, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 The parasite Schistocephalus solidus secretes 2 proteins with putative host manipulation functions 3 Chloé Suzanne Berger1,2,3, Jérôme Laroche2, Halim Maaroufi2, Hélène Martin1,2,4, 4 Kyung-Mee Moon5, Christian R. Landry1,2,4,6,7, Leonard J. Foster5 and Nadia Aubin- 5 Horth1,2,3 6 1Département de Biologie, Université Laval, Québec, QC, Canada 7 2Institut de Biologie Intégrative et des Systèmes (IBIS), Université Laval, Québec, QC, 8 Canada 9 3Ressources Aquatiques Québec (RAQ), Institut des sciences de la mer de Rimouski, 10 Québec, Canada 11 4Département de Biochimie, Microbiologie et Bioinformatique, Université Laval, Québec, 12 QC, Canada 13 5Department of Biochemistry & Molecular Biology, Michael Smith Laboratories, 14 University of British Columbia, Vancouver, Canada V6T 1Z4 15 6PROTEO, Le réseau québécois de recherche sur la fonction, la structure et l’ingénierie 16 des protéines, Université Laval, Québec, Canada 17 7Centre de Recherche en Données Massives (CRDM), Université Laval, Québec, 18 Canada 19 Corresponding author: Nadia Aubin-Horth ([email protected]) 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.02.03.932509; this version posted July 16, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

The Mallophaga of New England Birds James Edward Keirans Jr
University of New Hampshire University of New Hampshire Scholars' Repository Doctoral Dissertations Student Scholarship Spring 1966 THE MALLOPHAGA OF NEW ENGLAND BIRDS JAMES EDWARD KEIRANS JR. Follow this and additional works at: https://scholars.unh.edu/dissertation Recommended Citation KEIRANS, JAMES EDWARD JR., "THE MALLOPHAGA OF NEW ENGLAND BIRDS" (1966). Doctoral Dissertations. 834. https://scholars.unh.edu/dissertation/834 This Dissertation is brought to you for free and open access by the Student Scholarship at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. This dissertation has been microfilmed exactly as received 67—163 KEIRANS, Jr., James Edward, 1935— THE MALLOPHAGA OF NEW ENGLAND BIRDS. University of New Hampshire, Ph.D., 1966 E n tom ology University Microfilms, Inc., Ann Arbor, Michigan THE MALLOPHAGA OF NEW ENGLAND BIRDS BY JAMES E.° KEIRANS, -TK - A. B,, Boston University, i960 A. M., Boston University, 19^3 A THESIS Submitted to The University of New Hampshire In Partial Fulfillment of The Requirements for the Degree of Doctor of Philosophy Graduate School Department of Zoology June, 1966 This thesis has been examined and approved. May 12i 1966 Date ACKNOWLEDGEMENT I wish to express my thanks to Dr. James G. Conklin, Chairman, Department of Entomology and chairman of my doctoral committee, for his guidance during the course of these studies and for permission to use the facilities of the Entomology Department. My grateful thanks go to Dr. Robert L. -

Site Fidelity and Colony Dynaimcs of Caspian Terns Nesting at East
AN ABSTRACT OF THE THESIS OF Stefanie Collar for the degree of Master of Science in Wildlife Science presented on December 12, 2013. Title: Site Fidelity and Colony Dynamics of Caspian Terns Nesting at East Sand Island, Columbia River Estuary, Oregon, USA. Abstract approved: ____________________________________________________________________ Daniel D. Roby Fidelity to breeding sites in colonial birds is an adaptive trait thought to have evolved to enhance reproductive success by reducing search time for breeding habitat, allowing earlier nest initiation, facilitating mate retention, and reducing uncertainty of predator presence and food availability. Studying a seabird that has evolved relatively low colony fidelity, such as the Caspian tern, allowed me to explore the influence of stable nesting habitat on fidelity and nest site selection. The Caspian tern (Hydroprogne caspia) breeding colony on East Sand Island (ESI) in the Columbia River estuary is the largest colony of its kind in the world. This colony has experienced a decade of declining nesting success, culminating with the failure of the colony to produce any young in 2011. The objective of my study was to understand the dynamics of this Caspian tern super-colony by investigating the actions of breeding individuals over two seasons, as well as the behavior of the colony as a whole from 2001-2011. I was interested in (1) the degree of nest site fidelity exhibited by breeding terns in successive years and its relationship to reproductive success, and (2) how the interaction of top-down and bottom-up forces influenced average nesting success across the entire colony, and caused the observed trends in nesting success at the East Sand Island colony from 2001 to 2011. -

Incidence of a Haemoproteus Lari Parasitemia in a Threatened Goll
Ornis Fennica 72:159-164 1995 Incidence of a Haemoproteuslari parasitemia in a threatened Goll: Larus audouinii Xavier Ruiz, Daniel Oro & JacobGonzález-Solís Ruiz, X., Oro, D., González-Solís, J., Dept. de BiologiaAnimal (Vertebrats), Universitat : de Barcelona, Av. Diagonal 645. 08028-Barcelona, Spain ~ Received 15 August 1995, accepted 11 October 1995 Data on haematozoan parasiternias in Laridae are scarce and, since blood parasites may influence birds fitness, it is important to report their incidence in threatenedspecies such as Audouin' s Gull. Blood smearsof 90 Audouin' s Gulls caught during incubation at their two main breeding sites, fue Ebro Delta and fue Chafarinas Islands, were exarnined for Haemoproteus lari parasiternia. Overall prevalence of fue parasite was 92.1 % for fue Chafarinas Is. and 28.9% for fue Ebro Delta. To OUTknowledge, prevalences reported in ¡~' this study are fue highest ever recorded for Laridae. Differences in prevalence between localities were significant, but not differences between fue sexes within each locality. Males were more intenselyparasitized than femalesin Chafarinas,and gulls from Chafarinas were more intensely parasitized than those from fue Ebro Delta. Differences in intensity were also checked using data from sympatric Yellow-legged Gulls, L. cachinnans, and only fue factor locality was significant. There was no significant relationship betweenbody condition and intensity of parasiternia in males or females in either locality. These data indicate higher susceptibility to H. lari for gulls breeding at fue Chafarinas Islands. ~_. Introduction infections in wild birds are neededto evaluate their potential effects (Bennett et al. 1992, May 1995), The interaction ofhaematozoa and their avian hosts particularly in fue case of rafe or endangered spe- has received increasedattention since parasiteshave cies, as such Audouin' s Gull, one of fue few SPEC : been recognized as agents influencing many host species (Species ofEuropean Conservation Con- fitness components (reviews by M011eret al. -

SHOREBIRDS (Charadriiformes*) CARE MANUAL *Does Not Include Alcidae
SHOREBIRDS (Charadriiformes*) CARE MANUAL *Does not include Alcidae CREATED BY AZA CHARADRIIFORMES TAXON ADVISORY GROUP IN ASSOCIATION WITH AZA ANIMAL WELFARE COMMITTEE Shorebirds (Charadriiformes) Care Manual Shorebirds (Charadriiformes) Care Manual Published by the Association of Zoos and Aquariums in association with the AZA Animal Welfare Committee Formal Citation: AZA Charadriiformes Taxon Advisory Group. (2014). Shorebirds (Charadriiformes) Care Manual. Silver Spring, MD: Association of Zoos and Aquariums. Original Completion Date: October 2013 Authors and Significant Contributors: Aimee Greenebaum: AZA Charadriiformes TAG Vice Chair, Monterey Bay Aquarium, USA Alex Waier: Milwaukee County Zoo, USA Carol Hendrickson: Birmingham Zoo, USA Cindy Pinger: AZA Charadriiformes TAG Chair, Birmingham Zoo, USA CJ McCarty: Oregon Coast Aquarium, USA Heidi Cline: Alaska SeaLife Center, USA Jamie Ries: Central Park Zoo, USA Joe Barkowski: Sedgwick County Zoo, USA Kim Wanders: Monterey Bay Aquarium, USA Mary Carlson: Charadriiformes Program Advisor, Seattle Aquarium, USA Sara Perry: Seattle Aquarium, USA Sara Crook-Martin: Buttonwood Park Zoo, USA Shana R. Lavin, Ph.D.,Wildlife Nutrition Fellow University of Florida, Dept. of Animal Sciences , Walt Disney World Animal Programs Dr. Stephanie McCain: AZA Charadriiformes TAG Veterinarian Advisor, DVM, Birmingham Zoo, USA Phil King: Assiniboine Park Zoo, Canada Reviewers: Dr. Mike Murray (Monterey Bay Aquarium, USA) John C. Anderson (Seattle Aquarium volunteer) Kristina Neuman (Point Blue Conservation Science) Sarah Saunders (Conservation Biology Graduate Program,University of Minnesota) AZA Staff Editors: Maya Seaman, MS, Animal Care Manual Editing Consultant Candice Dorsey, PhD, Director of Animal Programs Debborah Luke, PhD, Vice President, Conservation & Science Cover Photo Credits: Jeff Pribble Disclaimer: This manual presents a compilation of knowledge provided by recognized animal experts based on the current science, practice, and technology of animal management. -

Pacific Seabirds
PACIFIC SEABIRDS A Publication of the Pacific Seabird Group Volume 36 Number 2 Fall 2009 PACIFIC SEABIRD GROUP Dedicated to the Study and Conservation of Pacific Seabirds and Their Environment The Pacific Seabird Group (PSG) was formed in 1972 due to the need for better communication among Pacific seabird researchers. PSG provides a forum for the research activities of its members, promotes the conservation of seabirds, and informs members and the public of issues relating to Pacific Ocean seabirds and their environment. PSG members include research scientists, conservation professionals, and members of the public from all parts of the Pacific Ocean. The group also welcomes seabird professionals and enthusiasts in other parts of the world. PSG holds annual meetings at which scientific papers and symposia are presented; abstracts for meetings are published on our web site. The group is active in promoting conservation of seabirds, include seabird/fisheries interactions, monitoring of seabird populations, seabird restoration following oil spills, establishment of seabird sanctuaries, and endangered species. Policy statements are issued on conservation issues of critical importance. PSG’s journals are Pacific Seabirds (formerly the PSG Bulletin) and Marine Ornithology. Other publications include symposium volumes and technical reports; these are listed near the back of this issue. PSG is a member of the International Union for Conservation of Nature (IUCN), the Ornithological Council, and the American Bird Conservancy. Annual dues for membership are $30 (individual and family); $24 (student, undergraduate and graduate); and $900 (Life Membership, payable in five $180 installments). Dues are payable to the Treasurer; see the PSG web site, or the Membership Order Form next to inside back cover.