Nitric Oxide Modulates Peristaltic Muscle Activity Associated with Fluid
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Coastal and Marine Ecological Classification Standard (2012)
FGDC-STD-018-2012 Coastal and Marine Ecological Classification Standard Marine and Coastal Spatial Data Subcommittee Federal Geographic Data Committee June, 2012 Federal Geographic Data Committee FGDC-STD-018-2012 Coastal and Marine Ecological Classification Standard, June 2012 ______________________________________________________________________________________ CONTENTS PAGE 1. Introduction ..................................................................................................................... 1 1.1 Objectives ................................................................................................................ 1 1.2 Need ......................................................................................................................... 2 1.3 Scope ........................................................................................................................ 2 1.4 Application ............................................................................................................... 3 1.5 Relationship to Previous FGDC Standards .............................................................. 4 1.6 Development Procedures ......................................................................................... 5 1.7 Guiding Principles ................................................................................................... 7 1.7.1 Build a Scientifically Sound Ecological Classification .................................... 7 1.7.2 Meet the Needs of a Wide Range of Users ...................................................... -

Bioluminescence and Fluorescence of Three Sea Pens in the North-West
bioRxiv preprint doi: https://doi.org/10.1101/2020.12.08.416396; this version posted December 9, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Bioluminescence and fluorescence of three sea pens in the north-west Mediterranean sea Warren R Francis* 1, Ana¨ısSire de Vilar 1 1: Dept of Biology, University of Southern Denmark, Odense, Denmark Corresponding author: [email protected] Abstract Bioluminescence of Mediterranean sea pens has been known for a long time, but basic parameters such as the emission spectra are unknown. Here we examined bioluminescence in three species of Pennatulacea, Pennatula rubra, Pteroeides griseum, and Veretillum cynomorium. Following dark adaptation, all three species could easily be stimulated to produce green light. All species were also fluorescent, with bioluminescence being produced at the same sites as the fluorescence. The shape of the fluorescence spectra indicates the presence of a GFP closely associated with light production, as seen in Renilla. Our videos show that light proceeds as waves along the colony from the point of stimulation for all three species, as observed in many other octocorals. Features of their bioluminescence are strongly suggestive of a \burglar alarm" function. Introduction Bioluminescence is the production of light by living organisms, and is extremely common in the marine environment [Haddock et al., 2010, Martini et al., 2019]. Within the phylum Cnidaria, biolumiescence is widely observed among the Medusazoa (true jellyfish and kin), but also among the Octocorallia, and especially the Pennatulacea (sea pens). -

Appendix C - Invertebrate Population Attributes
APPENDIX C - INVERTEBRATE POPULATION ATTRIBUTES C1. Taxonomic list of megabenthic invertebrate species collected C2. Percent area of megabenthic invertebrate species by subpopulation C3. Abundance of megabenthic invertebrate species by subpopulation C4. Biomass of megabenthic invertebrate species by subpopulation C- 1 C1. Taxonomic list of megabenthic invertebrate species collected on the southern California shelf and upper slope at depths of 2-476m, July-October 2003. Taxon/Species Author Common Name PORIFERA CALCEREA --SCYCETTIDA Amphoriscidae Leucilla nuttingi (Urban 1902) urn sponge HEXACTINELLIDA --HEXACTINOSA Aphrocallistidae Aphrocallistes vastus Schulze 1887 cloud sponge DEMOSPONGIAE Porifera sp SD2 "sponge" Porifera sp SD4 "sponge" Porifera sp SD5 "sponge" Porifera sp SD15 "sponge" Porifera sp SD16 "sponge" --SPIROPHORIDA Tetillidae Tetilla arb de Laubenfels 1930 gray puffball sponge --HADROMERIDA Suberitidae Suberites suberea (Johnson 1842) hermitcrab sponge Tethyidae Tethya californiana (= aurantium ) de Laubenfels 1932 orange ball sponge CNIDARIA HYDROZOA --ATHECATAE Tubulariidae Tubularia crocea (L. Agassiz 1862) pink-mouth hydroid --THECATAE Aglaopheniidae Aglaophenia sp "hydroid" Plumulariidae Plumularia sp "seabristle" Sertulariidae Abietinaria sp "hydroid" --SIPHONOPHORA Rhodaliidae Dromalia alexandri Bigelow 1911 sea dandelion ANTHOZOA --ALCYONACEA Clavulariidae Telesto californica Kükenthal 1913 "soft coral" Telesto nuttingi Kükenthal 1913 "anemone" Gorgoniidae Adelogorgia phyllosclera Bayer 1958 orange gorgonian Eugorgia -

Retinoic Acid and Nitric Oxide Promote Cell Proliferation and Differentially Induce Neuronal Differentiation in Vitro in the Cnidarian Renilla Koellikeri
Retinoic Acid and Nitric Oxide Promote Cell Proliferation and Differentially Induce Neuronal Differentiation In Vitro in the Cnidarian Renilla koellikeri Djoyce Estephane, Michel Anctil De´ partement de sciences biologiques and Centre de recherche en sciences neurologiques, Universite´ de Montre´ al, Case postale 6128, Succursale Centre-ville, Montre´ al, Que´ bec, Canada, H3C 3J7 Received 26 January 2010; revised 8 July 2010; accepted 9 July 2010 ABSTRACT: Retinoic acid (RA) and nitric oxide cell density. NO donors also induce cell proliferation in (NO) are known to promote neuronal development in polylysine-coated dishes, but induce neuronal differen- both vertebrates and invertebrates. Retinoic acid tiation and neurite outgrowth in uncoated dishes. No receptors appear to be present in cnidarians and NO other cell type undergoes differentiation in the pres- plays various physiological roles in several cnidarians, ence of NO. These observations suggest that in the sea but there is as yet no evidence that these agents have a pansy (1) cell adhesion promotes proliferation without role in neural development in this basal metazoan phy- morphogenesis and this proliferation is modulated pos- lum. We used primary cultures of cells from the sea itively by 9-cis RA and NO, (2) 9-cis RA and NO differ- pansy Renilla koellikeri to investigate the involvement entially induce neuronal differentiation in nonadherent of these signaling molecules in cnidarian cell differen- cells while repressing proliferation, and (3) the involve- tiation. We found that 9-cis RA induce cell prolifera- ment of RA and NO in neuronal differentiation tion in dose- and time-dependent manners in dishes appeared early during the evolutionary emergence of coated with polylysine from the onset of culture. -

Cladistic Analysis of the Pennatulacean Genus <I>Renilla</I
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UNL | Libraries University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Papers in Entomology Museum, University of Nebraska State January 2001 Cladistic analysis of the pennatulacean genus Renilla Lamarck, 1816 (Coelenterata, Octocorallia) Carlos D. Pérez Laboratorio de Biología de Cnidarios (LABIC), Departamento de Ciencias Marinas, Facultad de Ciencias Exactas y Naturales, UNMdP, Funes 3250, 7600, Mar del Plata, Argentina Federico C. Ocampo University of Nebraska - Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/entomologypapers Part of the Entomology Commons Pérez, Carlos D. and Ocampo, Federico C., "Cladistic analysis of the pennatulacean genus Renilla Lamarck, 1816 (Coelenterata, Octocorallia)" (2001). Papers in Entomology. 126. https://digitalcommons.unl.edu/entomologypapers/126 This Article is brought to you for free and open access by the Museum, University of Nebraska State at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Papers in Entomology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Published in Journal of Natural History 35:2 (January 2001), pp. 169–173; doi 10.1080/00222930150215305 Copyright © 2001 Taylor & Francis Ltd. Used by permission. http://www.tandf.co.uk/journals http://dx.doi.org/10.1080/00222930150215305 Accepted December 7, 1999. Cladistic analysis of the pennatulacean genus -

The Global Diversity of Sea Pens (Cnidaria: Octocorallia: Pennatulacea)
Review The Global Diversity of Sea Pens (Cnidaria: Octocorallia: Pennatulacea) Gary C. Williams* California Academy of Sciences, San Francisco, California, United States of America most brilliantly phosphorescent …. In some place nearly Abstract: Recent advances in deep-sea exploration everything brought up seemed full of luminous sparks. The technology coupled with an increase in worldwide biotic alcyonarians, the brittle-stars, and some annelids were the most surveys, biological research, and underwater photography brilliant. The Pennatulae, the Virgulariae, and the Gorgoniae shone in shallow water marine regions such as coral reefs, has with a lambient white light, so bright that it showed quite distinctly allowed for a relatively rapid expansion of our knowledge the hour on a watch …. We had another gorgeous display of in the global diversity of many groups of marine luminosity during this cruise …. The dredge came up tangle with organisms. This paper is part of the PLoS ONE review the long pink stems of the singular sea-pen Pavonaria quadrangularis. collection of WoRMS (the Worldwide Register of Marine Species), on the global diversity of marine species, and The Pavonariae were resplendent with a pale lilac phosphorescence treats the pennatulacean octocorals, a group of cnidarians like the flame of cyanogen gas: not scintillating …., almost commonly referred to as sea pens or sea feathers. This constant, sometimes flashing out at one point more brightly and also includes sea pansies, some sea whips, and various then dying gradually into comparative dimness, but always vermiform taxa. Pennatulaceans are a morphologically sufficiently bright to make every portion of a stem caught in the diverse group with an estimated 200 or more valid tangles or sticking to the ropes distinctly visible. -

Octocorallia: Pennatulacea)
SCIENTIA MARINA 83(3) September 2019, 261-276, Barcelona (Spain) ISSN-L: 0214-8358 https://doi.org/10.3989/scimar.04845.26A Resurrection of the sea pen genus Ptilella Gray, 1870 and description of Ptilella grayi n. sp. from the NE Atlantic (Octocorallia: Pennatulacea) Francisco J. García-Cárdenas 1, Jim Drewery 2, Pablo J. López-González 1 1 Biodiversidad y Ecología Acuática, Departamento de Zoología, Facultad de Biología, Universidad de Sevilla, Reina Mercedes 6, 41012 Sevilla, Spain. (FJG-C) (corresponding author) E-mail: [email protected]. ORCID-iD: https://orcid.org/0000-0002-1503-9552 (PJL-G) E-mail: [email protected]. ORCID-iD: https://orcid.org/0000-0002-7348-6270 2 Marine Scotland Science, Marine Laboratory, 375 Victoria Road, Aberdeen, Scotland, UK, AB11 9DB. (JD) E-mail: [email protected]. ORCID-iD: https://orcid.org/0000-0003-4308-1798 Summary: The order Pennatulacea covers a group of specialized and morphologically distinct octocorals found in all oceans from intertidal areas to more than 6000 m in depth. Sea pens constitute an important structural component in marine soft- bottom communities by increasing the complexity of these environments. Despite being both morphologically distinctive and ecologically important, the taxonomy and systematics of sea pens is still poorly understood. Recent molecular studies have shown the existence of convergent morphological features, making the current familial distribution of genera unstable. The genus Pennatula Linnaeus, 1758 was one of the first described octocoral genera. It is the type genus of its family, Pennatuli- dae. Colonies of this genus have a characteristic morphology. Recent sampling efforts in the northeastern Atlantic have pro- vided a number of colonies initially attributable to the genus Pennatula. -
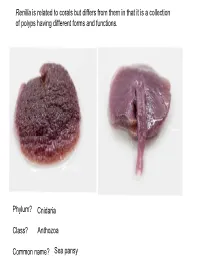
Phylum? Class? Common Name? Cnidaria Anthozoa Sea Pansy
Renilla is related to corals but differs from them in that it is a collection of polyps having different forms and functions. Phylum? Cnidaria Class? Anthozoa Common name? Sea pansy Phylum? Cnidaria Class? Hydrozoa Body form? Polyp Obelia colony Feeding polyp Phylum? Cnidaria Class? Hydrozoa Reproductive polyp Medusa bud – produced asexually Phylum? Cnidaria What is this? This is an Obelia medusa as seen under a compound microscope Class? Hydrozoa How does this reproduce? Sexually by producing eggs or sperm Phylum? Cnidaria Class? Hydrozoa What is the significance of the velum in the taxonomy of this organism? The velum is a characteristic of hydrozoan jellies. Thus Gonionemus is in Class Hydrozoa A B C D A: Exumbrella B: Subumbrella Goniomenus C: Manubrium D: Velum A: Tentacles C: Gonad A B B: Oral arm C Phylum? Cnidaria Identify Cells Class? Hydrozoa Physalia Tentacle Cnidocytes with nematocysts Phylum Cnidaria Class Hydrozoa 2. Identify structure within cell PhysaliaPhylum Tentacle Cnidaria Class Hydrozoa Nematocyst Physalia1. Identify Tentacle cell Cnidocytes with nematocysts Cnidocyte Phylum Cnidaria Class Hydrozoa Physalia Tentacle Cnidocytes with undischarged? Nematocysts Tentacle: Note Cnidocytes Bud (Asexual Reproduction) Phylum? Cnidaria Class Hydrozoa Hydra -Polyp Phylum Cnidaria Class Hydrozoa Obelia What stage of the lifecycle does this represent? Medusa stage (Sexually Reproduces) Phylum Cnidaria Class Hydrozoa Polpys Obelia Colony – A Colony of? Reproductive Polyp Asexual Stage Medusa Bud Feeding Polyp Phylum? Class? Common Name? Cnidaria Hydrozoa Portuguese Man-of-War Common Name? By-the-wind sailor or Velella Common name? Phylum? Class? A B Phylum? Cnidaria Class? Scyphozoa Common Name? Moon Jellly Phylum? Class? Common Name? Cnidaria Anthozoa Sea Anemone Kingdom? Animalia Phylum? Ctenophora Common name? Comb Jelly How does this animal move? Via cilia. -

Of Combined Demersal Fish and Megabenthic Invertebrate Recurrent Groups on the Southern California Shelf and Upper Slope, July-October, 2003
Southern California Bight 2003 Regional Monitoring Program: IV. Demersal Fishes and Megabenthic Invertebrates March 2007 M.J. Allen1, T. Mikel 2, D. Cadien3, J.E. Kalman4, E.T. Jarvis1, K.C. Schiff1, D.W. Diehl1, S.L. Moore1, S. Walther3, G. Deets5, C. Cash5, S. Watts6, D.J. Pondella II7, V. Raco-Rands1, C. Thomas4, R. Gartman8, L. Sabin1, W. Power3, A.K. Groce8 and J.L. Armstrong4 1Southern California Coastal Water Research Project 2Aquatic Bioassay and Consulting Laboratory 3County Sanitation Districts of Los Angeles County 4Orange County Sanitation District 5City of Los Angeles, Environmental Monitoring Division 6 Weston Solutions, Inc. 7Occidental College, Vantuna Research Group 8City of San Diego, Metropolitan Wastewater Department THE BIGHT '03 TRAWL WORKING GROUP MEMBERS Member Affiliation Chair - Dr. M. James Allen Southern California Coastal Water Research Project Co-Chair - Tim Mikel Aquatic Bioassay and Consulting Laboratories Dr. Jeff L. Armstrong Orange County Sanitation District Don Cadien County Sanitation Districts of Los Angeles County Curtis Cash City of Los Angeles, Environmental Monitoring Division Dr. Gregory Deets City of Los Angeles, Environmental Monitoring Division Dario W. Diehl Southern California Coastal Water Research Project Sarah Fangman Channel Islands National Marine Sanctuary Robin Gartman City of San Diego, Metropolitan Wastewater Department Ami K. Groce City of San Diego, Metropolitan Wastewater Department Erica T. Jarvis Southern California Coastal Water Research Project Dr. Julianne E. Kalman Orange County Sanitation District/University of California, Los Angeles/ currently California State University, Long Beach Shelly L. Moore Southern California Coastal Water Research Project Dr. Daniel J. Pondella, II Occidental College, Vantuna Research Group William Power County Sanitation Districts of Los Angeles County Valerie Raco-Rands Southern California Coastal Water Research Project Dr. -

Kahng Supplement
The following supplement accompanies the article Sexual reproduction in octocorals Samuel E. Kahng1,*, Yehuda Benayahu2, Howard R. Lasker3 1Hawaii Pacific University, College of Natural Science, Waimanalo, Hawaii 96795, USA 2Department of Zoology, George S. Wise Faculty of Life Sciences, Tel Aviv University, Ramat Aviv, Tel Aviv 69978, Israel 3Department of Geology and Graduate Program in Evolution, Ecology and Behavior, University at Buffalo, Buffalo, New York 14260, USA *Email: [email protected] Marine Ecology Progress Series 443:265–283 (2011) Table S1. Octocoral species and data used in the analysis and species assignment to taxonomic groups and to Clades and Subclades (Subcladecons: conservative subclade classification based only on genera in McFadden et. al. 2006) cons Clade Climate Latitude Location Subclade Sexuality Symbiont Subclade Sex ratio (F:M) Polyp fecundity Breeding period Max oocyte (um) Oogenesis (months) Group/Family Genus species Mode of reproduction References Alcyonacea Stolonifera Cornulariidae Cervera komaii 1 Japan 35 subtrop A G E 350 May-June Suzuki 1971 (Cornularia komaii) Cervera sagamiensis 1 Japan 35 subtrop A G E 630 Mar-June Suzuki 1971 (Cornularia sagamiensis) Clavulariidae Kahng et al. 2008; 1b 1c Hawaii 21 trop A G+ 1:1 S 550 <=12 7.4 continuous Carijoa riisei 1 Kahng 2006 Carijoa riisei 1 1b 1c Puerto Rico 18 trop A G+ 1:1 ? continuous Bardales 1981 (Telesto riisei) 1h Morocco 35 temp A ? E Benayahu & Loya Clavularia crassa 1 (Mediterranean) 1983; Benayahu 1989 GBR, 105 Alino & Coll 1989; 1n 1j 18 trop Z G E Oct-Nov Clavularia inflata 1 Phlippines 0 Bermas et al. 1992 11- Clavularia koellikeri 1 1n 1j GBR 12 trop Z ? B Bastidas et al 2002 Alcyoniina Alcyoniidae South Africa 27 subtrop ? G ? 200 Hickson 1900 Acrophytum claviger 1 0 Hartnoll 1975; Spain, France 1i 1g (NW 42 temp A G E June-July Garrabou 1999; Alcyonium acaule 1 McFadden 2001; E Mediterranean) Sala, pers. -

UC Santa Barbara UC Santa Barbara Electronic Theses and Dissertations
UC Santa Barbara UC Santa Barbara Electronic Theses and Dissertations Title Convergent evolution of eyes with divergent gene expression in jellyfish Permalink https://escholarship.org/uc/item/3gf789cz Author Picciani de Souza, Natasha Publication Date 2020 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California University of California Santa Barbara Convergent evolution of eyes with divergent gene expression in jellyfish A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Ecology, Evolution and Marine Biology by Natasha Picciani de Souza Committee in charge: Professor Todd H. Oakley, Chair Professor Celina E. Juliano, University of California Davis Professor Stephen R. Proulx December 2020 The dissertation of Natasha Picciani de Souza is approved. _____________________________________________ Prof. Stephen R. Proulx _____________________________________________ Prof. Celina E. Juliano, University of California Davis _____________________________________________ Prof. Todd H. Oakley, Committee Chair November 2020 Convergent evolution of eyes with divergent gene expression in jellyfish Copyright © 2020 by Natasha Picciani de Souza iii Acknowlegments I am sincerely grateful to Professor Todd Oakley for giving me the chance to pursue graduate school in one of the very best schools in the United States, for his patience and encouragement over all these years, for his immense support and, more than anything, for his empathy and trust during times of struggle. I am also very thankful to Professors Celina Juliano and Stephen Proulx for their very thoughtful suggestions that guided much of my research. To all my friends in the Oakley Lab, past and present, I am thankful for years of friendship, collegial support, coffee breaks, fun trips, memes, and scientific insights that significantly contributed to the work that I did. -

Taxonomic Revision of Leopold and Rudolf Blaschkas' Glass Models Of
http://www.natsca.org Journal of Natural Science Collections Title: Appendix to Taxonomic revision of Leopold and Rudolf Blaschkas’ Glass Models of Invertebrates 1888 Catalogue, with correction of authorities Author(s): Callaghan, E., Egger, B., Doyle, H., & E. G. Reynaud Source: Callaghan, E., Egger, B., Doyle, H., & E. G. Reynaud. (2020). Appendix to Taxonomic revision of Leopold and Rudolf Blaschkas’ Glass Models of Invertebrates 1888 Catalogue, with correction of authorities. Journal of Natural Science Collections, Volume 7, . URL: http://www.natsca.org/article/2587 NatSCA supports open access publication as part of its mission is to promote and support natural science collections. NatSCA uses the Creative Commons Attribution License (CCAL) http://creativecommons.org/licenses/by/2.5/ for all works we publish. Under CCAL authors retain ownership of the copyright for their article, but authors allow anyone to download, reuse, reprint, modify, distribute, and/or copy articles in NatSCA publications, so long as the original authors and source are cited. Callaghan, E., et al., 2020. JoNSC. 7. pp.34-43. Taxonomic revision of Leopold and Rudolf Blaschkas’ Glass Models of Invertebrates 1888 Catalogue, with correction of authorities Eric Callaghan1, Bernhard Egger2, Hazel Doyle1, and Emmanuel G. Reynaud1* 1School of Biomolecular and Biomedical Science, University College Dublin, University College Belfield, Dublin 4, Ireland. 2Institute of Zoology, University of Innsbruck, Austria Received: 28th June 2019 *Corresponding author: [email protected] Accepted: 3rd Feb 2020 Citation: Callaghan, E., et al. 2020. Taxonomic revision of Leopold and Rudolf Blaschkas’ Glass Models of Inverte- brates1888 catalogue, with correction of authorities. Journal of Natural Science Collections.