Pulmocare L.Pdf
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Supplementary Information
Supplementary Information Network-based Drug Repurposing for Novel Coronavirus 2019-nCoV Yadi Zhou1,#, Yuan Hou1,#, Jiayu Shen1, Yin Huang1, William Martin1, Feixiong Cheng1-3,* 1Genomic Medicine Institute, Lerner Research Institute, Cleveland Clinic, Cleveland, OH 44195, USA 2Department of Molecular Medicine, Cleveland Clinic Lerner College of Medicine, Case Western Reserve University, Cleveland, OH 44195, USA 3Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH 44106, USA #Equal contribution *Correspondence to: Feixiong Cheng, PhD Lerner Research Institute Cleveland Clinic Tel: +1-216-444-7654; Fax: +1-216-636-0009 Email: [email protected] Supplementary Table S1. Genome information of 15 coronaviruses used for phylogenetic analyses. Supplementary Table S2. Protein sequence identities across 5 protein regions in 15 coronaviruses. Supplementary Table S3. HCoV-associated host proteins with references. Supplementary Table S4. Repurposable drugs predicted by network-based approaches. Supplementary Table S5. Network proximity results for 2,938 drugs against pan-human coronavirus (CoV) and individual CoVs. Supplementary Table S6. Network-predicted drug combinations for all the drug pairs from the top 16 high-confidence repurposable drugs. 1 Supplementary Table S1. Genome information of 15 coronaviruses used for phylogenetic analyses. GenBank ID Coronavirus Identity % Host Location discovered MN908947 2019-nCoV[Wuhan-Hu-1] 100 Human China MN938384 2019-nCoV[HKU-SZ-002a] 99.99 Human China MN975262 -

1. Generic Names Levosalbutamol 0.25G Ambroxol 7.5Mg
1. Generic Names Levosalbutamol 0.25g Ambroxol 7.5mg Guaiphenesin 12.5 mg 2. Qualitative and Quantitative Composition Each ml contains Levosalbutamol 0.25g Ambroxol 7.5mg Guaiphenesin 12.5 mg 3. Dosage form and strength Oral drop solution containing Levosalbutamol 0.25g, Ambroxol 7.5mg, Guaiphenesin 12.5 mg. 4. Clinical particulars 4.1 Therapeutic indication Kofarest-PD drops is indicated for the treatment of productive cough associated with bronchospasm in conditions such as bronchitis and bronchial asthma as well as all conditions associated with tenacious mucus, wheezing and chest congestion. 4.2 Posology and method of administration The usual recommended dose of KOFAREST-PD Drops in children is: 1-2 years age: 0.8 ml thrice a day 2-3 years age: 1.2 ml thrice a day. 4.3 Contraindication Kofarest-PD drops are contraindicated in patients with hypersensitivity to any ingredient of the formulation. 4.4 Special warnings and precautions for use While treating cough as a symptom, it is important to make every effort to determine and treat appropriately the underlying cause, such as a specific infection. Caution should be observed while prescribing Kofarest-PD drops to children with hypertension, cardiovascular disease, uncontrolled juvenile diabetes mellitus, hyperthyroidism, and seizures or in patients who are unusually hypersensitive to sympathomimetic amines. 4.5 Drug interactions Hypokalaemia with high doses of ß2 -agonists may result in increased susceptibility to digitalis induced cardiac arrhythmias. Hypokalaemia may be enhanced by concomitant administration of aminophylline or other xanthine, corticosteroids or by diuretic therapy. Other sympathomimetic bronchodilators or epinephrine should not be used concomitantly with salbutamol, since their combined effect on the cardiovascular system may be deleterious to the patient. -

Supplementary Materials 07/09/2017
SMART Adolescent manuscript: Supplementary materials 07/09/2017 Supplementary materials Methods Definition and calculation of severe exacerbations The first three studies conducted 10-12, defined severe exacerbations as the need for oral corticosteroid (OCS) and/or hospitalisation/emergency room care due to asthma and/or a decrease in peak expiratory flow (PEF) of ≥30% on two consecutive days compared with the run-in period. In these studies, exacerbations were to be treated with a 10-day OCS course; an exacerbation lasting >10 days was considered a new exacerbation on the 11th day. Based on this experience, the later three studies 13-15 did not include falls in PEF in the exacerbation definition, nor this stipulated OCS treatment time, defining a severe exacerbation as the need for OCS for ≥3 days and/or hospitalisation/emergency room care due to asthma worsening. For the purpose of this analysis, we defined a severe exacerbation as the need for OCS (for ≥3 days 10-12) and/or hospitalisation/emergency room care due to asthma worsening; to align with this definition, data from 3 of the included studies 13-15 were reanalysed to exclude PEF fall from the exacerbation definition. 1 SMART Adolescent manuscript: Supplementary materials 07/09/2017 Literature review to identify any additional studies We conducted a review to identify any additional randomised controlled trials (RCTs) evaluating a combination of inhaled corticosteroids (ICS) and a rapid- acting bronchodilator in a single inhaler for both maintenance and as-needed relief of symptoms -

Sympathoadrenergic Modulation of Hematopoiesis: a Review of Available Evidence and of Therapeutic Perspectives
REVIEW published: 05 August 2015 doi: 10.3389/fncel.2015.00302 Sympathoadrenergic modulation of hematopoiesis: a review of available evidence and of therapeutic perspectives Marco Cosentino*, Franca Marino and Georges J. M. Maestroni Center for Research in Medical Pharmacology, University of Insubria, Varese, Italy Innervation of the bone marrow (BM) has been described more than one century ago, however the first in vivo evidence that sympathoadrenergic fibers have a role in hematopoiesis dates back to less than 25 years ago. Evidence has since increased showing that adrenergic nerves in the BM release noradrenaline and possibly also dopamine, which act on adrenoceptors and dopaminergic receptors (DR) expressed on hematopoietic cells and affect cell survival, proliferation, migration and engraftment ability. Remarkably, dysregulation of adrenergic fibers to the BM is associated with hematopoietic disturbances and myeloproliferative disease. Several adrenergic and dopaminergic agents are already in clinical use for non-hematological indications and with a usually favorable risk-benefit profile, and are therefore potential candidates for Edited by: non-conventional modulation of hematopoiesis. Wanda Lattanzi, Università Cattolica del Sacro Cuore, Keywords: dopamine, noradrenaline, adrenaline, adrenoceptors, dopaminergic receptors, hematopoiesis, Italy neuroimmune phamacology, drug repurposing Reviewed by: Sujit Basu, Introduction Ohio State University, USA Tsvee Lapidot, Weizmann Institute of Science, Israel The term ‘‘niche’’, derived from the Latin word ‘‘mytilus’’ (mussel), has eventually come to designate a shallow recess in a wall, as for a statue or other decorative object, in view of the *Correspondence: similarity with the shape of a seashell, and broadly a place suitable or appropriate for a person or Marco Cosentino, Center for Research in Medical thing. -

(12) Patent Application Publication (10) Pub. No.: US 2015/0202317 A1 Rau Et Al
US 20150202317A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0202317 A1 Rau et al. (43) Pub. Date: Jul. 23, 2015 (54) DIPEPTDE-BASED PRODRUG LINKERS Publication Classification FOR ALPHATIC AMNE-CONTAINING DRUGS (51) Int. Cl. A647/48 (2006.01) (71) Applicant: Ascendis Pharma A/S, Hellerup (DK) A638/26 (2006.01) A6M5/9 (2006.01) (72) Inventors: Harald Rau, Heidelberg (DE); Torben A 6LX3/553 (2006.01) Le?mann, Neustadt an der Weinstrasse (52) U.S. Cl. (DE) CPC ......... A61K 47/48338 (2013.01); A61 K3I/553 (2013.01); A61 K38/26 (2013.01); A61 K (21) Appl. No.: 14/674,928 47/48215 (2013.01); A61M 5/19 (2013.01) (22) Filed: Mar. 31, 2015 (57) ABSTRACT The present invention relates to a prodrug or a pharmaceuti Related U.S. Application Data cally acceptable salt thereof, comprising a drug linker conju (63) Continuation of application No. 13/574,092, filed on gate D-L, wherein D being a biologically active moiety con Oct. 15, 2012, filed as application No. PCT/EP2011/ taining an aliphatic amine group is conjugated to one or more 050821 on Jan. 21, 2011. polymeric carriers via dipeptide-containing linkers L. Such carrier-linked prodrugs achieve drug releases with therapeu (30) Foreign Application Priority Data tically useful half-lives. The invention also relates to pharma ceutical compositions comprising said prodrugs and their use Jan. 22, 2010 (EP) ................................ 10 151564.1 as medicaments. US 2015/0202317 A1 Jul. 23, 2015 DIPEPTDE-BASED PRODRUG LINKERS 0007 Alternatively, the drugs may be conjugated to a car FOR ALPHATIC AMNE-CONTAINING rier through permanent covalent bonds. -
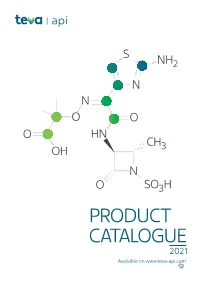
PRODUCT CATALOGUE 2021 Available on Product Known Teva Tech US EU Japan Korea Therapeutic Api IP File*** DMF DMF DMF DMF Area**
S NH2 N N O O O HN CH OH 3 N O SO3H PRODUCT CATALOGUE 2021 Available on www.teva-api.com Product Known Teva Tech US EU Japan Korea therapeutic api IP file*** DMF DMF DMF DMF area** A ABALOPARATIDE Osteoporosis ABEMACICLIB Oncology ABIRATERONE ACETATE Oncology ACALABRUTINIB Oncology AFATINIB Oncology ALCLOMETASONE DIPROPIONATE Dermatology ALLOPURINOL Rheumatology CEP AMCINONIDE Dermatology AMITRIPTYLINE HCl Neurology - Psychiatry CEP ANASTROZOLE Oncology CEP ANIDULAFUNGIN Infectious disease APALUTAMIDE* Oncology Coagulation APIXABAN inhibitors APREMILAST* Psoriasis ARIPIPRAZOLE* Neurology-Psychiatry CEP ATOMOXETINE HCl Neurology-Psychiatry ATORVASTATIN CALCIUM Lipid lowering CEP Preterm labor ATOSIBAN ACETATE prevention ATRACURIUM BESYLATE Muscle relaxant CEP AZACITIDINE Oncology AZITHROMYCIN DIHYDRATE Infectious disease CEP AZITHROMYCIN MONOHYDRATE Infectious disease CEP AZTREONAM* Infectious disease B BALOXAVIR Antiviral 2 Product Known Teva Tech US EU Japan Korea therapeutic api IP file*** DMF DMF DMF DMF area** BARICITINIB Rheumatology BECLOMETHASONE DIPROPIONATE Respiratory CEP BETAMETHASONE ACETATE Inflammation BETAMETHASONE BASE Dermatology CEP BETAMETHASONE DIPROPIONATE Dermatology CEP BETAMETHASONE VALERATE Dermatology CEP BICALUTAMIDE Oncology CEP BIVALIRUDIN Hematology BLEOMYCIN SULFATE Oncology BORTEZOMIB Oncology BREXANOLONE Neurology-Psychiatry BREXPIPRAZOLE Neurology-Psychiatry BROMOCRIPTINE MESYLATE Cardiovascular CEP BUDESONIDE Respiratory CEP BUPRENORPHINE BASE Neurology-Psychiatry CEP BUPRENORPHINE HCl Neurology-Psychiatry -

JMSCR Vol||07||Issue||08||Page 49-58||August 2019
JMSCR Vol||07||Issue||08||Page 49-58||August 2019 http://jmscr.igmpublication.org/home/ ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v7i8.08 An Observational Study of Drug Utilization Pattern and Pharmacovigilance of Infective Wheeze Associated Disorders in Children Authors Arthi S1*, Vanithasamuel2, Ramesh S3, Nirmala P4 1Post graduate, Department of Pharmacology, 2Professor, Department of Pharmacology 3Professor and Head, Department of Pediatrics, 4Professor and Head, Department of Pharmacology Rajah Muthiah Medical College, Chidambaram 608002 *Corresponding Author Arthi S Post graduate, Department of Pharmacology, Rajah Muthiah Medical College, Chidambaram 608002, India Abstract Background: Wheeze associated lower respiratory tract infections occurring world-wide are prevalent most commonly in childhood. Observational drug utilization studies help to evaluate, interpret and improve drug use as well as monitor the efficacy and safety of drugs prescribed. There was no previous documentation on drug utilization pattern of wheeze associated lower respiratory tract infections in the pediatric age group. Therefore, this study analyses the prescription pattern and adverse effects observed in such disorders in children attending the Pediatric outpatient department in a tertiary care hospital in Chidambaram. Materials and Methods: 100 prescriptions from the Pediatric department of Raja muthiah Medical College and hospital were analyzed. Data collected from parents or care givers relating to wheeze associated respiratory disorders was evaluated for prescription pattern, drug efficacy, adverse effect and compliance observed in polytherapy. Results: Majority of the children irrespective of severity received inhalation of β2 agonist levosalbutamol as a bronchodilator and sodium chloride (Nacl) nebulization compared to oral formulations in children. -

Dry Powder Inhalers Comprising a Carrier Other Than Lactose
(19) TZZ Z T (11) EP 2 682 097 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 08.01.2014 Bulletin 2014/02 A61K 9/00 (2006.01) A61K 9/14 (2006.01) A61K 31/40 (2006.01) A61K 31/439 (2006.01) (2006.01) (21) Application number: 13175077.0 A61K 31/4704 (22) Date of filing: 04.07.2013 (84) Designated Contracting States: • Türkyilmaz, Ali AL AT BE BG CH CY CZ DE DK EE ES FI FR GB 34460 Istanbul (TR) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO • Mutlu, Onur PL PT RO RS SE SI SK SM TR 34460 Istanbul (TR) Designated Extension States: BA ME (74) Representative: Sevinç, Erkan Istanbul Patent & Trademark (30) Priority: 05.07.2012 TR 201207842 Consultancy Ltd. Plaza 33, Buyukdere Cad. No: 33/16 (71) Applicant: Arven Ilac Sanayi Ve Ticaret A.S. Sisli Istanbul 34460 (TR) 34381 Istanbul (TR) (72) Inventors: • Cifter, Ümit 34460 Istanbul (TR) (54) Dry Powder Inhalers Comprising A Carrier Other Than Lactose (57) This invention relates to novel pharmaceutical other obstructive airways diseases. In addition, the compositions for inhalation comprising separately, se- present invention relates to novel pharmaceutical com- quentially or together, drugs having amine in the form of position for inhalation based on combinations of long act- a dry powder in admixture with a pharmaceutically ac- ing muscarinic antagonists, long acting beta agonists, ceptable carrier, other than lactose, and its use in the short acting beta-2 agonists, corticosteroids or a combi- treatment of respiratory condition selected from asthma nation of two or more of them. -
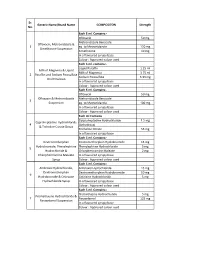
Paracetamol-Dextromethorphan-Hydrobromide-Phenylephrine-Hcl-Chlorpheniramine
Sr. Generic Name/Brand Name COMPOSITON Strength No. Each 5 ml. Contains:- Ofloxacin 50 mg Metronidazole Benzoate Ofloxacin, Metronidazole & 1 eq. to Metronidazole 120 mg Simethicone Suspension Simethicone 10 mg In a flavoured syrupy base Colour : Approved colour used Each 5 ml. contains:- Liquid Paraffin 1.25 ml Milk of Magnesia & Liquid Milk of Magnesia 3.75 ml 2 Paraffin and Sodium Picosulfate Sodium Picosulfate 3.33 mg Oral Emulsion In a flavoured syrupy base Colour : Approved colour used Each 5 ml. Contains:- Ofloxacin 50 mg Ofloxacin & Metronidazole Metronidazole Benzoate 3 Suspension eq. to Metronidazole 100 mg In a flavoured syrupy base Colour : Approved colour used Each ml Contains Cyrproheptadine Hydrochloride 1.5 mg Cyproheptadine Hydrochloride 4 (Anhydrous) & Trcholine Citrate Drops Tricholine Citrate 55 mg In a flavoured syrupy base Each 5 ml. Contains:- Dextromethorphan Dextromethorphan Hydrobromide 15 mg Hydrobromide, Phenylephrine Phenylephrine Hydrochloride 5 mg 5 Hydrochloride & Chlorpheniramine Maleate 2 mg Chlorpheniramine Maleate In a flavoured syrupy base Syrup Colour : Approved colour used Each 5 ml. Contains:- Ambroxol Hydrochloride, Ambroxol Hydrochloride 15 mg Dextromethorphan Dextromethorphan Hydrobromide 10 mg 6 Hydrobromide & Cetirizine Cetirizine Hydrochloride 5 mg Hydrochloride Syrup In a flavoured syrupy base Colour : Approved colour used Each 5 ml. Contains:- Promethazine Hydrochloride 5 mg Promethazine Hydrochloride & 7 Paracetamol 125 mg Paracetamol Suspension In a flavoured syrupy base Colour : Approved colour used Sr. Generic Name/Brand Name COMPOSITON Strength No. Each 5 ml. Contains:- Sodium Feredetate Sodium Feredetate, Eq. to Elemental Iron 33 mg 8 Cyanocobalamin & Folic Acid Cyanocobalamin 15 mcg Syrup Folic Acid 1.5 mg In a flavoured syrupy base Colour : Approved colour used Each 5 ml. -

1. Composition Each 5Ml Contains Levosalbutamol 0.5Mg Ambroxol 15Mg Guaiphenesin 50 Mg
1. Composition Each 5ml contains Levosalbutamol 0.5mg Ambroxol 15mg Guaiphenesin 50 mg 2. Dosage form and strength KOFAREST-LS Junior Syrup is available bottle of 60 ml. 3. Clinical particulars 3.1 Therapeutic indication KOFAREST-LS Junior Syrup is indicated for the treatment of productive cough associated with bronchospasm in conditions such as bronchitis and bronchial asthma as well as all conditions associated with tenacious mucus, wheezing and chest congestion. 3.2 Posology and method of administration The usual recommended dose of KOFAREST-LS Junior for: 2-5 years: 5 ml twice daily 6-12 years: 10 ml twice daily 3.3 Contraindication KOFAREST-LS Junior Syrup is contraindicated in patients with hypersensitivity to any ingredient of the formulation. 3.4 Special warnings and precautions for use While treating cough as a symptom, it is important to make every effort to determine and treat appropriately the underlying cause, such as a specific infection. Caution should be observed while prescribing KOFAREST-LS Syrup to children with hypertension, cardiovascular disease, uncontrolled juvenile diabetes mellitus, hyperthyroidism, and seizures or in patients who are unusually hypersensitive to sympathomimetic amines. 3.5 Drug interactions Hypokalaemia with high doses of ß2 -agonists may result in increased susceptibility to digitalis induced cardiac arrhythmias. Hypokalaemia may be enhanced by concomitant administration of aminophylline or other xanthine, corticosteroids or by diuretic therapy. Other sympathomimetic bronchodilators or epinephrine should not be used concomitantly with salbutamol, since their combined effect on the cardiovascular system may be deleterious to the patient. Salbutamol should be administered with caution in patients being treated with monoamine oxidase (MAO) inhibitors or tricyclic antidepressants, since the action of salbutamol on the vascular system may be potentiated. -

The Pulmonary Physician in Critical Care C 12: Acute Severe Asthma in the Intensive Care Unit P Phipps, C S Garrard
81 REVIEW SERIES Thorax: first published as 10.1136/thorax.58.1.81 on 1 January 2003. Downloaded from The pulmonary physician in critical care c 12: Acute severe asthma in the intensive care unit P Phipps, C S Garrard ............................................................................................................................. Thorax 2003;58:81–88 Most deaths from acute asthma occur outside hospital, within a year.25 Interestingly, the association but the at-risk patient may be recognised on the basis of between asthma deaths and β agonist use is still debated and there has been concern that the use prior ICU admission and asthma medication history. of long acting β agonists may increase asthma Patients who fail to improve significantly in the mortality.6 This has not been confirmed in studies emergency department should be admitted to an HDU monitoring their use.78In contrast, there is a con- sensus that underuse of anti-inflammatory treat- or ICU for observation, monitoring, and treatment. ment in the period leading up to the acute severe Hypoxia, dehydration, acidosis, and hypokalaemia attack worsens prognosis.9 render the severe acute asthmatic patient vulnerable to Unfortunately, a proportion of asthmatic pa- tients die despite reaching hospital alive. Such cardiac dysrrhythmia and cardiorespiratory arrest. deaths can usually be attributed either to Mechanical ventilation may be required for a small inadequate observation or treatment. Sadly, a proportion of patients for whom it may be life saving. number of patients suffer unobserved respiratory failure that progresses to cardiac arrest and Aggressive bronchodilator (continuous nebulised β anoxic brain damage.10 A small number of agonist) and anti-inflammatory therapy must continue patients are resistant to the most aggressive treatments and interventions. -

Guidelines for Diagnosis and Management of Bronchial Asthma: Joint ICS/NCCP (I) Recommendations
[Downloaded free from http://www.lungindia.com on Friday, July 22, 2016, IP: 14.139.56.194] Guidelines for diagnosis and management of bronchial asthma: Joint ICS/NCCP (I) recommendations Ritesh Agarwal, Sahajal Dhooria, Ashutosh Nath Aggarwal, Venkata N Maturu, Inderpaul S Sehgal, Valliappan Muthu, Kuruswamy T Prasad, Lakshmikant B Yenge, Navneet Singh, Digambar Behera, Surinder K Jindal, Dheeraj Gupta*, Thanagakunam Balamugesh, Ashish Bhalla, Dhruva Chaudhry, Sunil K Chhabra, Ramesh Chokhani, Vishal Chopra, Devendra S Dadhwal, George D’Souza, Mandeep Garg, Shailendra N Gaur, Bharat Gopal, Aloke G Ghoshal, Randeep Guleria, Krishna B Gupta, Indranil Haldar, Sanjay Jain, Nirmal K Jain, Vikram K Jain, Ashok K Janmeja, Surya Kant, Surender Kashyap, Gopi C Khilnani, Jai Kishan, Raj Kumar, Parvaiz A Koul, Ashok Mahashur, Amit K Mandal, Samir Malhotra, Sabir Mohammed, Prasanta R Mohapatra, Dharmesh Patel, Rajendra Prasad, Pallab Ray, Jai K Samaria, Potsangbam Sarat Singh, Honey Sawhney, Nusrat Shafiq, Navneet Sharma, Updesh Pal S Sidhu, Rupak Singla, Jagdish C Suri, Deepak Talwar, Subhash Varma Department of Pulmonary Medicine, Postgraduate Institute of Medical Education and Research (PGIMER), Indian Chest Society and National College of Chest Physicians, Chandigarh, Punjab, India *Author deceased after submission. Address for correspondence: Dr. Ritesh Agarwal, Department of Pulmonary Medicine, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh ‑ 160 012, Punjab, India. E‑mail: [email protected] Contents: 1. When should a diagnosis of asthma be considered? Executive Summary • A clinical diagnosis of asthma should be suspected A. Introduction in the presence of recurrent/episodic wheezing, B. Methodology breathlessness, cough, and/or chest tightness with no C.