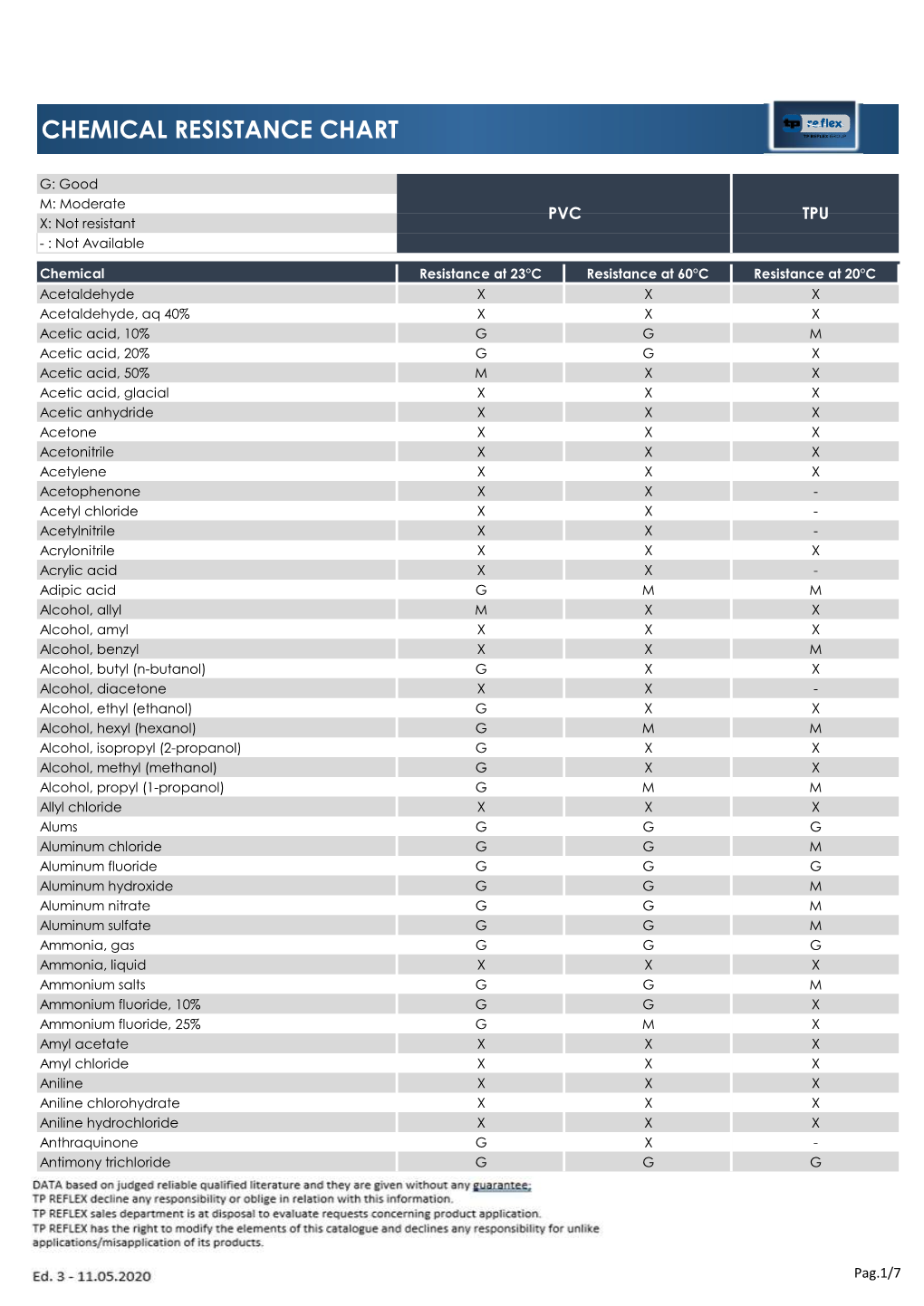
Chemical Resistance Chart
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Writing Total and Net Ionic Equations
WRITING TOTAL AND NET IONIC EQUATIONS http://www.csun.edu/~hcchm001/FreshChemHandouts.html 1. Write the overall equation including the correct designations for the physical state of the substances (s, l, g, aq). Balance this equation. Most of these kinds of equations are double displacement reactions: AX + BY 6 AY + BX 2. For the total ionic equations, write strong electrolytes in solution in the form of aqueous ions. (a) Strong acids. The common strong acids and their aqueous ions are: HI Hydroiodic acid H+-(aq) + I (aq) HBr Hydrobromic acid H+-(aq) + Br (aq) HCl Hydrochloric acid H+-(aq) + Cl (aq) +- HNO33Nitric acid H (aq) + NO (aq) +- HClO44Perchloric acid H (aq) + ClO (aq) +-2 H24SO Sulfuric acid 2 H (aq) + SO4(aq) (b) Strong bases. Strong bases are the hydroxides of the alkali (Group IA) and alkaline earth (Group IIA) metals ions which are sufficiently soluble. The common strong bases and their aqueous ions are: LiOH Lithium hydroxide Li+-(aq) + OH (aq) NaOH Sodium hydroxide Na+-(aq) + OH (aq) KOH Potassium hydroxide K+-(aq) + OH (aq) +2 - Sr(OH)2Strontium hydroxide Sr (aq) + 2 OH (aq) +2 - Ba(OH)2 Barium hydroxide Ba (aq) + 2 OH (aq) (c) Soluble salts. Determinations of the solubility of a salt may be made by reference to SOLUBILITIES OF IONIC COMPOUNDS. Soluble salts are written as their aqueous ions: NaCl(aq) Sodium chloride Na+-(aq) + Cl (aq) +-2 K24SO (aq) Potassium sulfate 2 K (aq) + SO4(aq) +-2 Li23CO (aq) Lithium carbonate 2 Li (aq) + CO3(aq) +-3 Na34PO (aq) Sodium phosphate 3 Na (aq) + PO4(aq) +-2 (NH42) SO4(aq) Ammonium sulfate 2 NH4(aq) + SO4 (aq) 3. -

Solid-Liquid Separation After Liquid-Liquid Extraction
CROATICA CHEMICA ACTA CCACAA 57 (2) 219-227 (1984) YU ISSN 0011-1643 CCA-1435 UDC 546. 73 + 543/545 Original Scientific Paper Solid-liquid Separation after Liquid-liquid Extraction: Spectrophotometric Determination of Cobalt by Extraction of its 2-Methoxyethyl Xanthate in Molten Naphthalene Mohammad F. Hussain, Rat K. Bansal, and Bal K. Puri* Chemistry Department, Indian I nstitute of Technology, New Delhi - 110 016, (India) Receiv ed July 19, 1983 A selective spectrophotometric method has been developed for the determination of cobalt after extraction of its 2-methoxyethyl xanthate complex into molten naphthalene. Cobalt reacts with this xanthate in the ratio of 1 : 2 (metal : ligand ratio) in a pH range of 3.5-9.2 and over an acid range of 2.5-7.0 M. It absorbs strongly at 355 nm. Beer's law is obeyed over the concentration range of 2.5-46.0 ~ig of cobalt in 10 ml of the final solution. The molar absorptivity and sensitivity in terms of Sandell's definition are calculated to be 1.287 x 104 1 mo1-1 cm-1 and 0.0046 µg /cm2 respecti vely. Ten replicate determinations of sample solution containing 25 µg of cobalt gives a mean absorbance of 0.546 with a standard deviation of ± 0.0037 and a relative standard deviation of ± 0.670/o. Interference by various ions has been studied and the conditions developed for the determination of cobalt in complex materials such as alloys. INTRODUCTION Several methods have been developed for the determination of cobalt using xanthates as a complex forming reagent. -

Guidelines for Using Perchloric Acid
Guidelines for Using Perchloric Acid Perchloric acid (HClO4) is a strong mineral acid. Under some circumstances it may act as an oxidizer and/or present an explosion hazards. These guidelines present information on how to handle and store perchloric acid safely. Please notify EH&S at 8-8182 if you are using perchloric acid in your laboratory. Using Perchloric Acid (< 72%) at Room Temperature At room temperature, perchloric acid up to concentrations of 72% has properties similar to other strong mineral acids. It is a highly corrosive substance and causes severe burns on contact with the eyes, skin, and mucous membranes. When used under these conditions, perchloric acid reacts as a strong non-oxidizing acid. The following precautions should be taken when using perchloric acid under these conditions: · Substitute with less hazardous chemicals when appropriate. Use dilute solutions (< 60%) whenever possible. · Conduct operations involving cold perchloric acid in a properly functioning chemical fume hood with current EH&S certification. If operations are conducted frequently or in large quantities contact EH&S to determine if a specially designed fume hood dedicated to perchloric acid use is required. · Always use impact-resistant chemical goggles, a face shield, neoprene gloves, and a rubber apron when handling perchloric acid. · When using or storing even dilute perchloric acid solutions avoid contact with strong dehydrating agents (concentrated sulfuric acid, anhydrous phosphorous pentoxide, etc.). These chemicals may concentrate the perchloric acid and make it unstable. · Always transfer perchloric acid over a sink or other suitable containment in order to catch any spills and afford a ready means of cleanup and disposal. -

Possible Atmospheric Origins of Perchlorate on Mars. Dc
40th Lunar and Planetary Science Conference (2009) 1567.pdf POSSIBLE ATMOSPHERIC ORIGINS OF PERCHLORATE ON MARS. D. C. Catling1,2, M. W. Claire3, R. C. Quinn4, K. J. Zahnle5, B. C. Clark6, S. Kounaves7, M. H. Hecht8. 1Dept. Earth Sciences, University of Bristol, Bristol, United Kingdom. 2Earth and Space Sciences/Astrobiology Program, University of Washington, Box 351310, Seattle WA 98195 ([email protected]). 3Dept. Astronomy/Astrobiology Program, University of Washington, Seattle WA 98195. 4SETI Institute/NASA Ames Research Center, Moffett Field, CA 94035. 5NASA Ames Research Center, Moffet Field, CA 94035. 6Space Exploration Systems, Lockheed Martin, Denver, CO 80201. 7Dept. of Chemistry, Tufts University, Medford, MA 02035. 8JPL/Caltech, Pasadena, CA 91109. Introduction: The wet chemistry experiment on playas [4]. Perchlorate is also found in carbonates of NASA’s Phoenix lander had cells where ~1 cm3 of the upper Eocene Mission Valley Formation of San Martian soil was mixed with 25 cm3 of dilute leaching Diego [5] and sylvinite (KCl) ore in central Canada solution and electrochemical measurements were made and Permo-Triassic New Mexico [4]. to identify a variety of soluble ions. Surprisingly, per- Isotopic analysis allows Atacama perchlorate to be - chlorate (ClO4 ) was measured as a dominant anion [1]. definitively attributed to an atmospheric source. Non- For an assumed soil sample mass ~1g, the soluble per- mass dependent (NMD) fractionation occurs in the - chlorate concentration converts to ~1wt% in the soil oxygen isotopes of ClO4 . This is ascribed to the pho- [1]. tochemical reaction of ozone with volatile chlorine These data suggest that most of the chlorine in the species because perchlorate inherits its distinctive soil at the Phoenix site has been oxidized to perchlo- NMD oxygen isotope signature from O3. -

Perchloric Acid Chemical Specific Standard Operating Procedure
Perchloric Acid Chemical Specific Standard Operating Procedure Perchloric Acid Corrosives - Strong Oxidizing Agents (SOA), Strong Acids (SA) & Potentially Explosive Compound (PEC) Areas with blue text indicate that information must be provided or modified by researcher prior to the SOP approval. This SOP is not a substitute for hands-on training. Print a copy and insert into your laboratory SOP binder. Department: Chemistry Date SOP was written: Monday, October 24, 2016 Date SOP was approved by PI/lab supervisor: Name: R. Sarpong Principal Investigator: Signature: ______________________________ Name: Melissa Hardy/Justin Jurczyk Internal Lab Safety Coordinator or Lab Manager: Lab Phone: 406-696-1225/412-728-1952 Office Phone: 510-642-6312 Name: Melissa Hardy/Justin Jurczyk Emergency Contact: Lab Phone: 406-696-1225/412-728-1952 Latimer Hall Location(s) covered by this SOP: 831,832,834,836,837,838,839,842,844,847,849 1 - Purpose This SOP covers the precautions and safe handling procedures for the use of Perchloric Acid. If you have questions concerning the applicability of any recommendation or requirement listed in this procedure, contact the Principal Investigator/Laboratory Supervisor or the campus Chemical Hygiene Officer at [email protected]. 2 - Physical & Chemical Properties/Definition of Chemical Group CAS#: 7601-90-3 Density: 1.664 g/mL at 25 °C (77 °F) Molecular Formula: HClO4 Flash point: 113 °C (235 °F) - closed cup Form: Liquid Lower explosion limit: no data available Color: colorless Upper explosion limit: no data available Melting point/freezing point: -18 °C (0 °F) Odor: no data available Rev. Date: 09Sept2016 1 Perchloric Acid Chemical Specific Standard Operating Procedure Boiling Point: 203 °C (397 °F) Odor threshold: no data available Vapor pressure: 9.1 hPa (6.8 mmHg) at 25 °C (77 °F) Perchloric acid is the inorganic compound with the formula HClO4. -

57(2)-08(Priyanka Singh 069).Fm
Korean Chem. Eng. Res., 57(2), 164-171 (2019) https://doi.org/10.9713/kcer.2019.57.2.164 PISSN 0304-128X, EISSN 2233-9558 Study of Protonation Behaviour and Distribution Ratios of Hydroxamic Acids in Hydrochloric and Perchloric Acid Solutions Through Hammett Acidity Function, Bunnett-Olsen and Excess Acidity Method Manisha Agarwal*, Priyanka Singh** and Rama Pande***,† *School of Studies in Chemistry, Pt. Ravishankar Shukla University, Raipur-492010, Chhattisgarh, India **Rungta College of Engineering & Technology, Bhilai-490024, Chhattisgarh, India ***Department of Chemistry, Govt. Digvijay PG Autonomous College,Rajnandgaon-491441 Chhattisgarh, India (Received 4 July 2018; Received in revised form 20 December 2018; accepted 21 December 2018) Abstract − The protonation parameters, dissociation constants (pKBH+) of conjugate acid, slope values (m, ϕ and m*) and correlation coefficients (r) of hydroxamic acids were determined by Hammett acidity function method, Bunnett- Olsen method and excess acidity method in hydrochloric and perchloric acid solutions. Effect of acid concentration on partition and percentage protonation was also studied. pKBH+ values show that hydroxamic acids do not behave as Ham- mett bases, but hydroxamic acids behave as weak bases in strong acidic solutions. The values of pKBH+ obtained through Bunnett-Olsen method and excess acidity method were compared with the Hammett acidity function. ChemAxon’s Mar- vinSketch 6.1.5 software was also used for determining pKa, pI and microspecies distribution (%) of hydroxamic acids with pH. Hydrogen donor and acceptor values and logD were also obtained. The results show that N-p-chlorophenyl-4- bromobenzohydroxamic acid has the highest pKa and lowest logD values. On the contrary, N-phenyl-3,5-dinitrobenzo- hydroxamic acid has lowest the pKa and highest logD values. -

INCOMPATIBLE CHEMICALS Up-Dated October 2011
INCOMPATIBLE CHEMICALS Up-dated October 2011 Sources Accident Prevention Manual for Industrial Operations, 6th ed., National Safety Council, Fire Protection Guide on Hazardous Materials, 6th ed., National Fire Protection Association; 49CFR173; recent laboratory inspections. Incompatible materials should not be stored together where they can be inadvertently mixed or where a spill or leak can cause danger. General guidelines are: 1. Oxygen and fuels must not be stored together. 2. Water reactive materials are not to be stored with flammables (except where a flammable is used to blanket a material such as sodium and then at least practical quantity), or in an area where they could become wet (under a sink, sprinkler head, shower, etc.) 3. Strong acids and bases are not to be stored together. 4. Materials which can produce poisonous gases must not be stored with products which accelerate the release of the gas. (Examples: cyanogens are not to be stored with an acid, or cleaning products containing chlorine are not to be stored with ammonia.) 5. Explosives (picric acid, etc.) are not to be stored with fuels. 6. Incompatible acids must not be stored together. (Examples: perchloric acid is not to be stored with a reducing agent such as sulfuric acid, as upon mixing, this could produce a shock sensitive explosive; nitric acid and acetic acid, a potential explosive mixture, must not be stored together.) Specific examples of incompatible items likely to be found in laboratories are: Chemical Store Away From or Out of Contact With Acetic Acid Chromic acid, nitric acid, hydroxyl compounds, ethylene glycol, perchloric acid, peroxides and permanganates. -

Decomposition of Coal with Periodic Acid Plus Perchloric Acid for the Isolation of Spores and for the Determination of Minor Components Gerald I
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1963 Decomposition of coal with periodic acid plus perchloric acid for the isolation of spores and for the determination of minor components Gerald I. Spielholtz Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Analytical Chemistry Commons, and the Oil, Gas, and Energy Commons Recommended Citation Spielholtz, Gerald I., "Decomposition of coal with periodic acid plus perchloric acid for the isolation of spores and for the determination of minor components " (1963). Retrospective Theses and Dissertations. 2499. https://lib.dr.iastate.edu/rtd/2499 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. This dissertation has been 63—7276 microfilmed exactly as received SPIELHOLTZ, Gerald I., 1937- DECOMPOSITION OF COAL WITH PERIODIC ACID PLUS PERCHLORIC ACID FOR THE ISOLATION OF SPORES AND FOR THE DETERMINATION OF MINOR COMPONENTS. Iowa State University of Science and Technology Ph.D„ 1963 Chemistry, analytical University Microfilms, Inc., Ann Arbor, Michigan DECOMPOSITION OF COAL WITH PERIODIC ACID PLUS PERCHLORIC ACID FOR THE ISOLATION OF SPORES AND FOR THE DETERMINATION OF MINOR COMPONENTS by Gerald I. Spielholtz A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OF PHILOSOPHY Major Subject: Analytical Chemistry Approved: Signature was redacted for privacy. -

"Perchloric Acid and Perchlorates". In: Kirk-Othmer Encyclopedia Of
PERCHLORIC ACID AND PERCHLORATES 1. Introduction Perchloric acid [7601-90-3], HClO4, is one of the strongest of the mineral acids. The perchlorates are more stable than the other chlorine oxyanions, ie, chlorates, À À À ClO 3; chlorites, ClO 2; or hypochlorites, OCl (3) (see DICHLORINE MONOXIDE, HYPOCHLOROUS ACID, AND HYPOCHLORITES). Essentially, all of the commercial perchlo- rate compounds are prepared either directly or indirectly by electrochemical oxi- dation of chlorine compounds (4–8) (see CHLORINE; ELECTROCHEMICAL PROCESSING, INTRODUCTION). The perchlorates of practically all the electropositive metals are known, except for a few cations having low charges. The most outstanding property of the perchlorates is their oxidizing ability. On heating, these compounds decompose into chlorine, chlorides, and oxygen gas. Aqueous perchlorate solutions exhibit little or no oxidizing power when dilute or cold. However, hot concentrated perchloric acid is a powerful oxidizer and whenever it contacts oxidizable matter extreme caution is required. The acidified concentrated solutions of perchlorate salts must also be handled with caution. Ammonium perchlorate [7790-98-9] (AP) is one of the most important perchlorates owing to its high (54.5%) O2 content and the absence of residue on decomposition. These properties, along with a long shelf life, make it a useful rocket propellant (see EXPLOSIVES AND PROPELLANTS) (9). AP is a true explosive as demonstrated by the explosion at the PEPCON plant at Henderson, Nevada, in 1988 (10). Following early (1890s) work (11), France, Germany, Switzerland, and the United States began to produce perchlorates for use as propellants and explo- sives. Whereas total world production of perchlorates did not exceed 1800 t/yr until 1940, it increased dramatically during World War II to about 18,000 t/yr in order to supply the rocket and missile industries. -

Proceedings of the Indiana Academy of Science
Acidity of Trichloroacetic Acid Howard Burkett, Richard Murphy and Dean Yarian, DePauw University Authors of numerous textbooks state that trichloroacetic acid is a strong acid—comparable to the strong mineral acids. This statement is correct for dilute aqueous solutions. In addition to other evidence measurements of the Hammett acidity functioni showed that the acidity function, H , Ho = pK a + log([B] / [BH+]) (1) of trichloroacetic acid was almost identical with that of the strong mineral acids in dilute solutions. At concentrations above about twenty percent, however, the acidity function did not increase further but remained constant with increasing acid concentration. The maximum value of the acidity function was reported to be approximately +0.60. Two observations would lead one to question this low value for the acidity of trichloroacetic acid. First, comparison of the rates for the hydrolysis of sucrose vs. the acidity function for various acids (2) shows that trichloroacetic acid appears to be a much better catalyst than the mineral acids. If the rate constants vs. molar concentration (rather than vs. acidity function) are plotted, the curve for trichloro- acetic acid deviates no more from the mineral acids than the mineral acids deviate from each other. If catalytic action is proportional to acidity function, as was proposed for the mineral acids, it seems likely that the measurements of the acidity functions were in error. That only one indicator was used in this study made this possibility of error more plausible. Second, cryoscopic measurements (3) show that trichloroacetic acid is unionized (as a base) in pure sulfuric acid. On the other hand, weak to moderately strong acids (acetic, chloroacetic, dichloroacetic, nitric and hydrochloric acids, for example) do ionize as bases in sulfuric acid. -

Perchloric Acid Safety Guidelines
PERCHLORIC ACID SAFETY GUIDELINES Perchloric acid is a strong mineral acid commonly used as a laboratory reagent. It is a clear, colorless liquid with no odor. Most perchloric acid is sold as solutions of 60% to 72% (w/w) acid in water. Perchloric acid is considered one of the strongest superacids. It is highly reactive with metals, dangerously corrosive and readily forms explosive mixtures. Anyone who works in laboratories containing perchloric acid should familiarize themselves with its SDS and a clear Standard Operating Procedure (SOP) should be established. Therefore, careful precautions should always be taken when handling this chemical. This document discusses the properties, health and safety hazards, how to properly handle and store perchloric acid. Also included are emergency procedures for dealing with accidental perchloric acid contact, including first aid treatment information. WARNING: In addition to being a corrosive liquid, while not combustible, under some circumstances perchloric acid may act as an oxidizer and present an explosion hazards. Organic materials are especially susceptible to spontaneous combustion if mixed or contacted with perchloric acid. Under some circumstances, perchloric acid vapors form perchlorates in ductwork, which are shock sensitive. 1. Properties Names: perchloric acid; hydronium perchlorate; dioxonium perchlorate usually sold in solutions of 60-72% (w/w) in water; anhydrous perchloric acid (>85% w/w) Chemical Formula: HClO4 CAS #: 7601-90-3 Physical aspect (solution 72% w/w): Colorless liquid, oily Odorless to slight chlorine smell Very hygroscopic and water soluble EHS-DOC-010 v.2 1 / 9 Table 1. Physical and Toxicological Properties of Perchloric Acid (60-72% w/w) Molar Mass 100.46 g mol-1 Boiling point 203°C Melting point -20°C Vapor pressure 6.8 mm Hg at 25°C Density 1.67g·cm−3 pKa (water) ≈-8 PEL (TWA) N/A IDHL N/A 2. -

Ozone Chemistry in Aqueous Solution Ozone Chemistry
Ozone chemistry in aqueous solution ---Ozone-Ozone decomposition and stabilisation Margareta Eriksson Licentiate Thesis Department of Chemistry Royal Institute of Technology Stockholm, Sweden, 2005 Ozone chemistry in aqueous solution –Ozone decomposition and stabilisation Margareta Eriksson Licentiate Thesis Department of Chemistry Royal Institute of Technology Stockholm, Sweden, 2004 TRITA-OOK-1078 ISSN 0348-825X AKADEMISK AVHANDLING Som med tillstånd av Kungliga Tekniska Högskolan i Stockholm framlägges till offentlig granskning för avläggande av Teknologie Licentiatexamen i Kemi den 13 maj 2005, kl. 10:30 i H1, Teknikringen 33, Stockholm. Margareta Eriksson, April 2005 Universitetsservice US AB, Stockholm 2005 Hobbes: Do you have an idea for your story yet? Calvin: No, I'm waiting for inspiration. You can't just turn on creativity like a faucet. You have to be in the right mood. Hobbes: What mood is that? Calvin: Last-minute panic. Bill Watterson, The Calvin and Hobbes Tenth Anniversary Book Abstract Ozone is used in many applications in the industry as an oxidising agent for example for bleaching and sterilisation. The decomposition of ozone in aqueous solutions is complex, and is affected by many properties such as, pH, temperature and substances present in the water. Additives can either accelerate the decomposition rate of ozone or have a stabilising effect of the ozone decay. By controlling the decomposition of ozone it is possible to increase the oxidative capacity of ozone. In this work the chemistry of acidic aqueous ozone is studied and ways to stabilise the decomposition of ozone in such solutions. The main work emphasizes the possibility to use surfactants in order to develop a new type of cleaning systems for the sterilisation of medical equipment.