Article Download
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
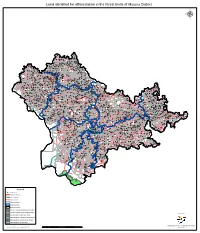
Land Identified for Afforestation in the Forest Limits of Mysuru District Μ
Land identified for afforestation in the forest limits of Mysuru District µ Bettahalli Guluvina Attiguppe Hosuru Hosuru Mudiguppe Munjanahalli Mundur Kallikoppalu Dodda Koppalu Kanchanahalli Chikka Bherya Kallikoppalu Tandre Salekoppalu Tandrekoppalu Sankanahalli Somanahalli Bherya Melur Haradanahali Bachahalli Badiganahalli Madapura Katnalu Kurubarahalli Sugganahalli Abburu Doddadahalli Ramapura Mudlabidu Elemuddanahalli Harumballikoppalu Basavanahalli Kallimuddanahalli Karatalu Lakkikuppe Yaladahalli Kodiyala Manugonahalli Hebsuru Harumballi Lakkikuppekoppalu Ankanahalli Kanagalu Saligrama Hosa Agrahara Sarguru Hale Mirle Tandere Gayanahalli Honnenahalli Kottayanakoppalu Cummanahalli Natnahalli Hanumanahalli Dodda Vaddaragudi Honnapura Alchanahalli Dodda Hanasoge Kanchinakere Pasupati Kakanahalli Subbayyanakoppalu Kedaga Chiknayakanahalli Adaguru PasupatikoppaluMavanuru Mirle Chikka Vaddaragudi Rajanabilugull Bichanahalli Galigekere Balluru C Koppalu Malnayakanahalli Ganganahalli Arjunahalli KD Kambipura Yaremanuganahalli Koluru Ganganahallikoppalu Chikka Hanasage Kaggala Adaguru Halaganahalli Chennamgere Ganganakuppe Kavalanahalli Bandahalli Kalyanapura Sannegaudanakoppalu Dammanahalli Byalakuppe Suragahalli Hadya Baluru Doddakoppalu Harinahalli Sakkare Dod Kamarhalli Kumarikoppalu Karadipura Nadappanahalli K.R.Nagar Badakanakoppalu Anivalu Meluru Halgegaudankoppalu Baluru Chapparadahalli Sangarashettihalli Maraduru Kanaganahalli Ambalare Dammanahallikoppalu Diddahalli Chunchanakatte Chik Kamarhalli Manchinahalli Maradur Koppalu Mayigaudanahalli -
IGF-Hemanth [Compatibility Mode]
KARNATAKA STATE SDI – AN OVERVIEW Karnataka NRDMS program • Initiated NRDMS program in 1992-93 • Joint initiative of Department of Science & Technology (Government of Karnataka and Government of India) • Established district spatial data centers in all the districts to support development planning using GIS. • Karnataka is the 1 st state institutionalise spatial data for decision-making. DATA FROM STATISTICAL TOPOSHEETS NRDMS METHODOLOGY TABLES AERIAL / THEMATIC SATELLITE MAPS DATA PROCESSING DECISION THEMATIC TO COMPUTER DISTRICT CHARTS/ PROCESSING SUPPORT DECISION MAPS COMPATIBLE DATABASE DIAGRAMS FORMATS SYSTEM CENSUS INPUT TO REPORTS MODELS OTHER GENERATED / SECONDARY ANALYSED SOURCES MAPS LIMITED USER-ORIENTED APPLICATION COLLECTION PREPROCESSING STORAGE ANALYSIS PRIMARY RETRIEVALS MODELLING SURVEYS INPUTS PROCESSING STAGES OUTPUTS AGENCIES NRDMS USERS National/State Survey and Mapping Organisations • Survey of India • Indian Meteorological Department • Census of India • Geological Survey of India • National Bureau of Soil Sciences & Land -use Board • Forest Survey of India • National Remote Sensing Establishment • Research Data – publications, reports etc. • State Remote Sensing Centre‘s • Survey Settlement and Land Records • Google/GPS – being used for updation and data capture Spatial data is stored on Survey of India framework State/District Data • Agro-Climatic Zones • Drainage • Taluk/District Boundary • Basin Boundary • District Head Quarters • • Division Boundary Catchment Boundary • Division Head Quarters • Sub catchment Boundary • Geology • Subdivision Boundary • Meteorological Station • Sub watershed Boundary • Parliamentary • Tanks Constituencies • National Highway • Legislative Assembly Constituencies • State Highway • Rail network • Major district road • Reservoir • Other District Roads • River • Village roads Highlights: •Value added services & customized applications based on user needs both at district/state level. •Developed a standardized seamless spatial data for storing, sharing, access and discovery through Karnataka Geo-portal . -

Mysore District Is an Administrative District Located in the Southern Part of the State of Karnataka, India
Chapter-1 Mysore District Profile Mysore District is an administrative district located in the southern part of the state of Karnataka, India. The district is bounded by Mandya district to the northeast, Chamrajanagar district to the southeast, Kerala state to the south,Kodagu district to the west, and Hassan district to the north. It features many tourist destinations, from Mysore Palace to Nagarhole National Park. This district has a prominent place in the history of Karnataka; Mysore was ruled by the Wodeyars from the year 1399 till the independence of India in the year 1947. Mysore's prominence can be gauged from the fact that the Karnatakastate was known previously as Mysore state. It is the third most populous district in Karnataka (out of 30), after Bangaloreand Belgaum. Geography Mysore district is located between latitude 11°45' to 12°40' N and longitude 75°57' to 77°15' E. It is bounded by Mandya district to the northeast, Chamrajanagar district to the southeast, Kerala state to the south, Kodagu district to the west, andHassan district to the north. It has an area of 6,854 km² (ranked 12th in the state). The administrative center of Mysore District is Mysore City. The district is a part of Mysore division. Prior to 1998, Mysore district also contained theChamarajanagar district before that area was separated off. The district lies on the undulating table land of the southern Deccan plateau, within the watershed of the Kaveri River, which flows through the northwestern and eastern parts of the district. The Krishna Raja Sagara reservoir, which was formed by building a dam across the Kaveri, lies on the northern edge of the district. -

KRISHNARAJANAGARA Polling Station (PS) Polling Station Name PS Location No Name of the BLO Mobile No
Contact Details of Booth Level Officers BLOs Ac. No. and Name : 211-KRISHNARAJANAGARA Polling Station (PS) Polling Station Name PS Location No Name of the BLO Mobile No. No Govt Higher Primary School 1 - Govt Higher Primary 1 Swaminayaka 8197049118 Bettahalli School,Bettahalli Govt Higher Primary School 2 - Govt Higher Primary 2 Nagarathna M E 8277551109 ,Maluganahalli School,Maluganahalli Govt Higher Primary School 3 - Govt Higher Primary 3 Vindyashree 9606616393 Doddakoppalu School,Doddakoppalu Govt Higher Primary School 4 - Govt Higher Primary 4 Bhadraiah 9901604180 ,Thandre Ankanahalli School,tandre Ankanahalli Govt Higher Primary School 5 - Govt Higher Primary 5 T S Veerbadrappa 9448940754 ,Kuppahalli School,Kuppahalli Govt Higher Primary School 6 - Govt Higher Primary 6 Jayakumar 9741935656 Mudiguppe School,Mudi Guppe Govt Higher Primary School, 7 (Room No-1) Guluvina Rangaswamy Y R 9632724015 Attiguppe 7 - Govt Higher Primary Govt Higher Primary School, School,Guluvina Attiguppe 8 (Room No.2), Guluvina Chithrashekar 8722700898 Attiguppe Govt Lower Primary School 8 - Govt Lower Primary 9 Kumarashetty 9743547092 Hosuru School,Hosuru Govt Lower Primary School, 9 - Govt Lower Primary 10 Balakrishna 9164118236 Chikkabherya School,Chikkabherya Govt Higher Primary School 10 - Govt Higher Primary 11 Sumithra 7411306364 ,Munjanahalli School,Munjanahalli Govt Lower Primary School 11 - Govt Lower Primary 12 K.P.Krishnegowda 7399087593 Gerudada School,Gerudada Govt Higher Primary School 12 - Govt Higher Primary 13 Chikkegowda H D 8496823242 -

Sub Centre List As Per HMIS SR
Sub Centre list as per HMIS SR. DISTRICT NAME SUB DISTRICT FACILITY NAME NO. 1 Bagalkote Badami ADAGAL 2 Bagalkote Badami AGASANAKOPPA 3 Bagalkote Badami ANAVALA 4 Bagalkote Badami BELUR 5 Bagalkote Badami CHOLACHAGUDDA 6 Bagalkote Badami GOVANAKOPPA 7 Bagalkote Badami HALADURA 8 Bagalkote Badami HALAKURKI 9 Bagalkote Badami HALIGERI 10 Bagalkote Badami HANAPUR SP 11 Bagalkote Badami HANGARAGI 12 Bagalkote Badami HANSANUR 13 Bagalkote Badami HEBBALLI 14 Bagalkote Badami HOOLAGERI 15 Bagalkote Badami HOSAKOTI 16 Bagalkote Badami HOSUR 17 Bagalkote Badami JALAGERI 18 Bagalkote Badami JALIHALA 19 Bagalkote Badami KAGALGOMBA 20 Bagalkote Badami KAKNUR 21 Bagalkote Badami KARADIGUDDA 22 Bagalkote Badami KATAGERI 23 Bagalkote Badami KATARAKI 24 Bagalkote Badami KELAVADI 25 Bagalkote Badami KERUR-A 26 Bagalkote Badami KERUR-B 27 Bagalkote Badami KOTIKAL 28 Bagalkote Badami KULAGERICROSS 29 Bagalkote Badami KUTAKANAKERI 30 Bagalkote Badami LAYADAGUNDI 31 Bagalkote Badami MAMATGERI 32 Bagalkote Badami MUSTIGERI 33 Bagalkote Badami MUTTALAGERI 34 Bagalkote Badami NANDIKESHWAR 35 Bagalkote Badami NARASAPURA 36 Bagalkote Badami NILAGUND 37 Bagalkote Badami NIRALAKERI 38 Bagalkote Badami PATTADKALL - A 39 Bagalkote Badami PATTADKALL - B 40 Bagalkote Badami SHIRABADAGI 41 Bagalkote Badami SULLA 42 Bagalkote Badami TOGUNSHI 43 Bagalkote Badami YANDIGERI 44 Bagalkote Badami YANKANCHI 45 Bagalkote Badami YARGOPPA SB 46 Bagalkote Bagalkot BENAKATTI 47 Bagalkote Bagalkot BENNUR Sub Centre list as per HMIS SR. DISTRICT NAME SUB DISTRICT FACILITY NAME NO. -
14Th FC 1St Installment of Basic Grants to Gps 2015-16 - MYSURU
14th FC 1st Installment of Basic Grants to GPs 2015-16 - MYSURU District Name Taluk Name GP Code GP Name Bank Details Branch Name IFSC Code Bank Account GP Share (in Rs) Number MYSURU- HEGGADADEVANKOTE- Cauvery kalpataru Grameena 1522001001 ALANAHALLI Kyathanahalli SBMY0RRCKGB 13034054995 678695.72 ೖಸೂರು –ೆಗಡ ೇವನೋೆ Bank MYSURU- HEGGADADEVANKOTE- Cauvery kalpataru Grameena 1522001014 ANNURU Annur SBMY0RRCKGB 13099063992 675910.51 ೖಸೂರು –ೆಗಡ ೇವನೋೆ Bank MYSURU- HEGGADADEVANKOTE- Cauvery kalpataru Grameena 1522001028 ANTHARASANTHE Antharasanthe SBMY0RRCKGB 13007109518 560658.04 ೖಸೂರು –ೆಗಡ ೇವನೋೆ Bank MYSURU- HEGGADADEVANKOTE- Cauvery kalpataru Grameena 1522001025 B.MATAKERE B.matakere SBMY0RRCKGB 13021058576 1279223.83 ೖಸೂರು –ೆಗಡ ೇವನೋೆ Bank MYSURU- HEGGADADEVANKOTE- Cauvery kalpataru Grameena 1522001027 BEECHANAHALLI Beechanahalli SBMY0RRCKGB 13040098311 603200.60 ೖಸೂರು –ೆಗಡ ೇವನೋೆ Bank MYSURU- HEGGADADEVANKOTE- Cauvery kalpataru Grameena 1522001015 BHEEMANAHALLI Annur SBMY0RRCKGB 13099064000 423220.93 ೖಸೂರು –ೆಗಡ ೇವನೋೆ Bank MYSURU- HEGGADADEVANKOTE- Cauvery kalpataru Grameena 1522001026 BIDARAHALLI Beechanahalli SBMY0RRCKGB 13040098388 827216.83 ೖಸೂರು –ೆಗಡ ೇವನೋೆ Bank MYSURU- HEGGADADEVANKOTE- Cauvery kalpataru Grameena 1522001013 CHAKKODANAHALLI H.D.Kote SBMY0RRCKGB 13042128596 1175949.92 ೖಸೂರು –ೆಗಡ ೇವನೋೆ Bank MYSURU- HEGGADADEVANKOTE- Cauvery kalpataru Grameena 1522001004 CHIKKEREYOORU H.D.Kote SBMY0RRCKGB 13042128620 507720.76 ೖಸೂರು –ೆಗಡ ೇವನೋೆ Bank MYSURU- HEGGADADEVANKOTE- Cauvery kalpataru Grameena 1522001032 D.B.KUPPE Antharasanthe SBMY0RRCKGB -

Krishnarajanagara Assembly Karnataka Factbook
Editor & Director Dr. R.K. Thukral Research Editor Dr. Shafeeq Rahman Compiled, Researched and Published by Datanet India Pvt. Ltd. D-100, 1st Floor, Okhla Industrial Area, Phase-I, New Delhi- 110020. Ph.: 91-11- 43580781-84 Email : [email protected] Website : http://www.indiastatelections.com Online Book Store : www.indiastatpublications.com Report No. : AFB/KA-211-0121 ISBN : 978-93-87130-93-7 First Edition : January, 2018 Third Updated Edition : January, 2021 Price : Rs. 11500/- US$ 310/- © Datanet India Pvt. Ltd. All rights reserved. No part of this book may be reproduced, stored in a retrieval system or transmitted in any form or by any means, mechanical photocopying, photographing, scanning, recording or otherwise without the prior written permission of the publisher. Please refer to Disclaimer at page no. 200 for the use of this publication. Printed in India Contents No. Particulars Page No. Introduction 1 Assembly Constituency - (Vidhan Sabha) at a Glance | Features of Assembly 1-2 as per Delimitation Commission of India (2008) Location and Political Maps Location Map | Boundaries of Assembly Constituency - (Vidhan Sabha) in 2 District | Boundaries of Assembly Constituency under Parliamentary 3-10 Constituency - (Lok Sabha) | Town & Village-wise Winner Parties- 2019, 2018, 2014, 2013 and 2009 Administrative Setup 3 District | Sub-district | Towns | Villages | Inhabited Villages | Uninhabited 11-19 Villages | Village Panchayat | Intermediate Panchayat Demographics 4 Population | Households | Rural/Urban Population -

Page 1 DISTRICT NAME SCHOOL NAME and ADDRESS BANGALORE NORTH GOVERNMENT JUNIOR COLLEGE for BOYS 18TH CROSS MALLESWARAM BANGALORE
Page 1 DISTRICT NAME SCHOOL NAME AND ADDRESS GOVERNMENT JUNIOR COLLEGE FOR BOYS 18TH CROSS MALLESWARAM BANGALORE NORTH BANGALORE NORTH [email protected], [email protected], [email protected],[email protected] ,[email protected], [email protected], [email protected], BANGALORE NORTH CORPORATION BOYS HIGH SCHOOL PILLANNA GRDN BANGALORE NORTH [email protected],[email protected],[email protected],[email protected], [email protected] , [email protected], BANGALORE NORTH CORPORATION HIGH SCHOOL BROADWAY BANGALORE NORTH [email protected] BANGALORE NORTH CORPORATION HIGH SCHOOL MALLESHWARAM BANGALORE NORTH [email protected], [email protected], [email protected], [email protected], [email protected], [email protected] , [email protected],[email protected], BANGALORE NORTH CORPORATION HIGH SCHOOL TASKER TOWN BANGALORE NORTH BANGALORE NORTH ST.JOSEPHS INDIAN HIGH SCHOOL 23 GRANT ROAD BANGALORE NORTH [email protected], [email protected], [email protected],[email protected],[email protected], R.B.A.N.M.S HIGH SCHOOL (MAIN) NO. 30 DICKENSON ROAD BANGALORE BANGALORE NORTH NORTH [email protected],[email protected], [email protected] ,[email protected] , QUWATHUL ISLAM HIGH SCHOOL NO.8 BOREBANK RD BENSON TOWN BANGALORE NORTH BANGALORE NORTH [email protected] GANDHINAGAR HIGHER SECONDARY SCHOOL KUMARA PARK WEST BANGALORE NORTH EXTENSION BANGALORE NORTH BANGALORE -

Sl. No Name Address District Written Test Marks out Off 100 42 56 a T Ankesha a T Ankesha S-O Thimmanna Mustur (V) (P) Jagalur
|| Lº §° î®±ºc±w¯s¯‡®± w®î®±» || §° £µ°q®ä u®Áš®Ú¡® S¯ä¯†ÃvÜ ‡µ²°cwµ †.› h䚬Ô(‹) Nµ°ºu®äN®Zµ°‹, u®Áš®Ú¡® HŒ¯å A„®ãtÁS®¢Sµ, vw¯ºN® 16.06.2019Š®0u®± w®lµu® îµ±°ªæX¯Š®N®/ N®Zµ°‹ š®œ¯‡®±N®/w®S®u®± š®œ¯‡®±N® œ®±uµÛ‡®± wµ°Š® wµ°î®±N¯r‡®± ªRq® y®‹°£µ‡®± y®ªq¯ºý® D Nµ¡®w®ºruµ. Cu®Š®ªå 50 î®±q®±Ù 50 Oʺq® œµX®±Ï AºN® y®lµu®î®Š®w®±Ý î®±±ºvw® œ®ºq®u® š®ºu®ý®Áw®NµÊ B‡µ±Ê ¯l®Œ¯Tu®±Û, š®ºu®ý®Áw®u® š®Ú¡® î®±q®±Ù vw¯ºN®î®w®±Ý ¬šµ¶g¬w®ªå œ¯N®Œ¯S®±î®¼u®± î®±q®±Ù y®äqµã°N®î¯T Hš¬.Hº.Hš¬ î®±²©N® ¯Ÿr x°l®Œ¯S®±î®¼u®±. HŒ¯å A„®ãtÁS®¢Sµ A†Ãw®ºu®wµS®¡®±. Sl. Written test Marks Name Address District No out off 100 W/O SUDHEER SRIVATSA HOUSE, PADIPILLYA 1 ASMITHA PILLYA POST AND VILLAGE Ujire 42 BELTHANGADY TALUK D.K, KARNATAKA-574217 S/O GIDDAPPA PUJAR, AT: HATTI (L) 2 ANJANEYA Koppal 56 POST: KALAKERI, TQ/DIST: KOPPAL -583230 A T Ankesha S-o Thimmanna 3 A T Ankesha Mustur (v) (p) Jagalur Taluk Davanagere 50 Davanagere dist 8th Ward Siddlinga Nagar 4 Aalima B Koppal 38 Karatagi 5 Abdhul hameed S/o Abubakar Shorapur Yadagiri YADAGIRI 52 STATION ROAD OPP URDU 6 ABDUL KHADAR ,A, HANAGI SCHOOL GADAG Gadag 38 S/O FAYAZ AHAMED HARALAHALLI HARIHARA(T) 7 ABDUL RAHAMAN H F Davanagere 45 DAVANGERE(D) HALIVANA(P) PIN CODE:577530 S/O BABY POOJARY BUDENGOTTU HOUSE MALADY VILLAGE 8 ABHIJITH B Ujire 58 PUNJALAKATTE POST BELTHANGADYTQ, D.K KARNATAKA-574233 S/O CHANDRAHASA D ABHISHEK NILAYA DHARMASTHALA POST AND 9 ABHILASH C VILLAGE, Ujire 47 BELTHANAGADY (TQ) DAKSHINA KANNADA 574216 S/O VISHWANATHA H S 10 ABHILASH H V WARD#12 ANANDAIAH EXTENSION Thumkur -

Karnataka AIM for Seva San Francisco Bay Area’S Commitment to Karnataka
Karnataka AIM for Seva San Francisco Bay Area’s Commitment to Karnataka AIM For Seva Free Student Hostel for Boys Chunchunakatte Village, Hosuru Post, K.R. Nagar Taluk, Mysore District – 571 617 Karnataka Mysore District Know Chunchunakatte Chunchunakatte is a small village in Krishnarajanagara taluk in Literacy Rate Mysore district of Karnataka state, India with a total of 1,056 families residing. It comes under Kuppe panchayat and belongs to Mysore division. It is located 50 km towards west from the district 100 head quarter, Mysore and 170 km from the state capital, Bengaluru. 80 79.21% Gudduganahalli (8.8 km), Somanahalli (11.3 km), Benaganahalli 68.20% (13.2 km), Kogilur (12.2 km), Chibkahalli (14 km), Kestur (4.5 km), 60 Kanchagarakoppalu (8.4 km), Nijaganahalli (12.3 km), Mavathur 56.98% Hantha (4 km) are the nearby villages to Chunchunakatte. 40 Chunchunakatte is surrounded by Krishnarajpet taluk in the east, Hunsur taluk in the south, Holenarsipur taluk in the north and 20 Piriyapatna taluk in the west. Shrirangapattana, Mysore, Nanjangud, Hassan are the nearby cities to Chunchunakatte. Literacy Male Female Total population – 4,363 Children between the age group of 0-6 years - 360 Average Sex Ratio of Chunchunakatte – 975 Know Mysore Number of villages in Mysore District : 1,362 Total population of Mysore District : 3,001,127 Male population of Mysore District : 1,511,600 Female population of Mysore District : 1,489,527 The average literacy rate is 72.79% Your Impact Take 13-year-old Sangamesh Prabhu Burli from Bagalkot town. He has a hearing impairment, but is a confident young boy who aspires to become a drama actor. -
Mysore District Lists
Group "C" Societies having less than Rs.10 crores of working capital / turnover, Mysore District lists. Sl No Society Name Mobile Number Email ID District Taluk Society Address 1 Agricultural Co-Operative Society, Mysore HEGGADADEVANKOTE - -- Annuru 2 Belladakuppe Mahadeshwaraswamy Mysore HEGGADADEVANKOTE - Kallukutikara Labour Contract Co- -- Operative Society, Saraguru 3 Bheemanakolli Mysore HEGGADADEVANKOTE - Mahadeshwaraswamy Labour -- Contract Co-Operative Society, Vaddarapalya 4 Bhuvaneshwari JFCS, Mysore HEGGADADEVANKOTE - -- Kollegowdanahalli 5 Bidarahalli Multipurpose Co- Mysore HEGGADADEVANKOTE - -- Operative Society, Bidarahalli 6 Chikkadevamma Kallukutikara Mysore HEGGADADEVANKOTE - Labour Contract Co-Operative -- Society, Vaddaragudi 7 Chikkadevamma Women Mysore HEGGADADEVANKOTE - Multipurpose Co-Operative Society, -- Saraguru 8 Cotton Weavers Co-Operative Mysore HEGGADADEVANKOTE - -- Society,saraguru 9 Dr.Ambedkar Fishermen Co- Mysore HEGGADADEVANKOTE - -- Operative Society, Hand post 10 Kabini Colony Girijanara Fishers Co- Mysore HEGGADADEVANKOTE - Operative Society, Kabini Colony - - 11 Kalikambha Kallikutikara Rural Indu. Mysore HEGGADADEVANKOTE - Co-Operative Society, Beeranahalli - - 12 Karnataka state govt. Employees Mysore HEGGADADEVANKOTE - House building Co-Operative Society - - 13 KITTURU PACS - - Mysore HEGGADADEVANKOTE - 14 Kolagala Fishemen Co-Operative Mysore HEGGADADEVANKOTE - -- Society, 15 lamps Co-operativeerative SOCIETY Mysore HEGGADADEVANKOTE H.D KOTE TALUK MYSORE DIST ,Pin : 571114 -- ltdl H.D.Kote