B. Tech. Chemical Technology - Plastic Technology Semester 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UNDER GRADUATE PROGRAMME B.Tech
CURRICULUM UNDER GRADUATE PROGRAMME B.Tech. NATIONAL INSTITUTE OF TECHNOLOGY KARNATAKA, SURATHKAL SRINIVASNAGAR PO, MANGALORE – 575 025 KARNATAKA, INDIA Phone: +91-824-2474000 Web-Site: www.nitk.ac.in Fax : +91-824 –2474033 2012 NATIONAL INSTITUTE OF TECHNOLOGY KARNATAKA, SURATHKAL ---------------------------------------------------------- MOTTO * Work is Worship VISION * To Facilitate Transformation of Students into- Good Human Beings, Responsible Citizens and Competent Professionals, focusing on Assimilation, Generation and Dissemination of Knowledge. MISSION * Impart Quality Education to Meet the Needs of Profession and Society and Achieve Excellence in Teaching-Learning and Research. * Attract and Develop Talented and Committed Human Resource and Provide an Environment Conducive to Innovation, Creativity, Team-spirit and Entrepreneurial Leadership * Facilitate Effective Interactions Among Faculty and Students and Foster Networking with Alumni, Industries, Institutions and Other Stake-holders. * Practice and Promote High Standards of Professional Ethics, Transparency and Accountability. CURRICULUM UNDER GRADUATE PROGRAMMES 2012 NATIONAL INSTITUTE OF TECHNOLOGY KARNATAKA, SURATHKAL ---------------------------------------------------------- CURRICULUM 2012 UNDER GRADUATE PROGRAMME B.Tech. SECTIONS 1. Regulations (General) 2. Regulations – UG 3. Forms & Formats – UG 4. Course Structure – UG 5. Course Contents – UG ------------------------------------------------------------------------------- NITK- UG- Curriculum 2012 NATIONAL INSTITUTE -

Håltavla För Upphängning Av Irislights
Håltavla för upphängning av Irislights Produktutveckling av uppfästningskonstruktion för LED-slinga Framework for fastening of Irislights Product development for attachment of LED-trail Rebecca Magyar Fakulteten för hälsa-, teknik- och naturvetenskap Högskoleingenjörsprogrammet i innovationsteknik och design Examensarbete 22,5 hp Monica Jakobsson Leo de Vin 2016-06-20 Blanka sidor finns med i rapporten för att nya kapitel och huvudrubriker skall hamna på framsidan av blad med dubbelsidiga utskrifter. Sammanfattning Rapporten behandlar ett examensarbete för högskoleingenjörsprogrammet inom innovationsteknik och design, utfört av Rebecca Magyar. Uppdragsgivare för projektet var Home Structures Sweden AB och arbetet riktade sig till det egna varumärket Irislights med Peter Nordin som handledare på företaget. Irislights är en produkt som består av en LED-slinga för inomhusbruk. Produkten är tänkt att vara kreativ för kunden färg- och formmässigt vilket låter kunderna själva kombinera handgjorda bomullsbollar som fästs kring en diod på slingan. Projektets huvudsakliga uppgift var att generera en lösning för fästning av befintlig produkt med avseende på kundernas behov i att hänga upp slingan. Den valda lösningen ska ge kunden utrymme för att utnyttja sin egen kreativitet. Arbetet utgick ifrån designprocessen där en förstudie var inledande fas för arbetets uppbyggnad. Förstudien innefattade en kartläggning av kommande trender inom inrednings- och belysningsbranschen, undersökning av kundernas behov och reflekterande arbete kring framtidens inredningsuttryck. Undersökning av teknik ingick även i förstudien för att få mer information kring befintlig produkt samt krav denna kom att ställa på det framtagna koncept som rapporten berör. Eftersom nuvarande produkt och den framtagna upphängningskonstruktionen ska samverka med varandra kommer uppställda krav vara av betydelse för utformningen. -
National Institute of Technology Silchar
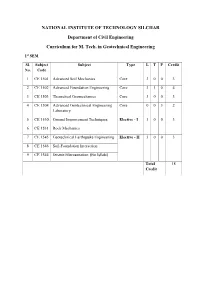
NATIONAL INSTITUTE OF TECHNOLOGY SILCHAR Department of Civil Engineering Curriculum for M. Tech. in Geotechnical Engineering 1st SEM Sl. Subject Subject Type L T P Credit No. Code 1 CE 1501 Advanced Soil Mechanics Core 3 0 0 3 2 CE 1502 Advanced Foundation Engineering Core 3 1 0 4 3 CE 1503 Theoretical Geomechanics Core 3 0 0 3 4 CE 1504 Advanced Geotechnical Engineering Core 0 0 3 2 Laboratory 5 CE 1530 Ground Improvement Techniques Elective - I 3 0 0 3 6 CE 1531 Rock Mechanics 7 CE 1545 Geotechnical Earthquake Engineering Elective - II 3 0 0 3 8 CE 1546 Soil-Foundation Interaction 9 CE 1544 Seismic Microzonation (No Syllabi) Total 18 Credit Advanced Soil Mechanics L T P C CE 1501 3 0 0 3 Pre-requisites: Graduate in Civil Engineering Detailed Syllabus Introduction to Soil Mechanics Formation of soil, Identification and classification of soil, Soil weight volume relationship, Index properties of soils. Introduction to Soil Mechanics Formation of soil, Identification and classification of soil, Soil weight volume relationship, Index properties of soils. Surface Tension and Capillary phenomenon Measurement of capillary rise in soil, Soil moisture, Soil –water potential, Buckingham’s concept, Component potential, Measurement of soil-water potential, Tentiometer, Pressure- plate apparatus. Permeability of Soil Permeability, Darcy’s law, Kozeny- Carmans’s equation, Theories of wells, flow nets and their properties. Seepage Analysis Seepage, Quick sand & critical hydraulic condition, Flow net in dams, Flow net by relaxation method, Seepage forces, Uplift, Piping phenomenon, Problems, Effective and Pore water pressures. Compressibility and Consolidation Compressibility and Consolidation, I-D & 3-D consolidation problems, Settlement of soil due to consolidation. -

WMCAUS 2016 Will Be One of the Annual Series
Preface This Abstract Collection consists of the abstracts of papers presented in the “World Multidisciplinary Civil Engineering-Architecture-Urban Planning Symposium” in the city of romance Prague (Czech Republic) during 13-17 June 2016. The World Multidisciplinary Civil Engineering-Architecture-Urban Planning Symposium (WMCAUS) aims to provide a forum for discussion of the latest findings and technologies in different fields of Civil Engineering, Architecture and Urban Planning, to give opportunities for future collaborations, to be a platform for sharing knowledge and experiences in the fields of Civil Engineering, Architecture and Urban Planning, to lead for providing a forum for early career researchers for presentation of their work and discussion of their ideas with experts in different fields of Civil Engineering, Architecture and City and Urban Planning such as; Construction Management and Engineering, Construction Materials, Geotechnics, Hydromechanics, Structural Engineering, Building Performance Simulations, Transportation, Architectural Space, Social Sciences and Architecture, Architectural Culture, Theories of Vision and Visuality, Architectural Design and Methods, Architectural Historiography, Architectural Heritage and Conservation of Historical structures, Sustainability in the Built Environment, Urban Planning, Public Space, Urban Design, Theories and Methods, Regional Planning, Archaeological Method and Theories, Sustainable Urban Development, Urban Sociology, Economics and Politics, Risk Management and Mitigation Planning, -

New Materials and IT Technologies in Production Engineering
NEW MATERIALS ANANANDAND IT TECHNOLOGIES IN PRODUCTION ENGINEERING NEW MATERIALS ANANANDAND IT TECHNOLOGIES IN PRODUCTION ENGINEERING Editors: Antoni Świć, Jerzy Lipski LUBLIN 2011 REVIEWERS: Prof. Eng. Wiktor Taranenko Prof. Eng. Jerzy Stamirowski Typeset by: M. Sc. Eng. Tomasz Kusz The content-related, responsibility, style and presentation form falls on the authors of individual contributions. Wydanie publikacji dofinansowane przez Ministra Nauki i Szkolnictwa Wy szego © Copyright by Lubelskie Towarzystwo Naukowe Lublin 2011 No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form, or by any means, electronic, photocopying or otherwise, without the prior written permission if the Publishers. ISBN 978-83-62-025-14-5 WYDAWCA: Lubelskie Towarzystwo Naukowe Plac Litewski 2, 20-080 Lublin DRUK: Wydawnictwo-Drukarnia „Liber-Duo” ul. Długa 5, 20-346 Lublin CONTENTS JANUSZ W. SIKORA, BRONISŁAW SAMUJŁO The effectiveness of the single screw extrusion of selected thermoplastic polymers ........................................................................................................ 7 ANETA TOR – WI¡ TEK Issues of poly(vinyl chloride) extrusion process modified by blowing agent 18 JAKUB MATUSZAK, KAZIMIERZ ZALESKI Effect of milling parameters upon burr formation during AZ91 HP magnesium alloy face milling ....................................................................... 31 TADEUSZ HEJWOWSKI, JANUSZ D¡ BSKI Application of arc weld surfacing for reclamation of knives used in recycling of -

Regulations 2017 Curriculum and Syllabi B.Tech
REGULATIONS 2017 CURRICULUM AND SYLLABI B.TECH. AUTOMOBILE ENGINEERING Regulation 2017 Department of Automobile Engineering, B.S.A.C.I.S.T 1 VISION AND MISSION OF THE INSTITUTION VISION B.S. Abdur Rahman Institute of Science and Technology aspires to be a leader in Education, Training and Research in Engineering, Science, Technology and Management and to play a vital role in the socio-Economic progress of the Country. MISSION • To blossom into an internationally renowned University • To empower the youth through quality education and to provide professional leadership • To achieve excellence in all its endeavors to face global challenges • To provide excellent teaching and research ambience • To network with global institutions of excellence, Business, Industry and Research Organizations • To contribute to the knowledge base through scientific enquiry, Applied research and Innovation Regulation 2017 Department of Automobile Engineering, B.S.A.C.I.S.T 2 DEPARTMENT OF AUTOMOBILE ENGINEERING VISION AND MISSION VISION To be a leader for Education, Training, Consultancy and Research in Automobile Engineering for the progress of Automotive Industries and over-all Socio-Economic progress of the Country in a sustainable manner. MISSION • To provide quality education to the students and to mould them as professionals with sound knowledge in the field of Automobile Engineering. • To equip students to solve challenging problems in Automobile Engineering and related areas taking in to account their impact on the society. • To facilitate students to develop good communication, leadership and managerial skills through team approach in conducting experiments and projects • To pursue academic and collaborative research with industry and related research institutions. -

B.Tech. Polymer.Pmd
UNIVERSITY VISION AND MISSION VISION B.S. Abdur Rahman Institute of Science & Technology aspires to be a leader in Education, Training and Research in Engineering, Science, Technology and Management and to play a vital role in the Socio-Economic progress of the Country. MISSION • To blossom into an internationally renowned University • To empower the youth through quality education and to provide professional leadership • To achieve excellence in all its endeavors to face global challenges • To provide excellent teaching and research ambience • To network with global Institutions of Excellence, Business, Industry and Research Organizations • To contribute to the knowledge base through Scientific enquiry, Applied Research and Innovation B.Tech. Polymer Engineering 2 VISION AND MISSION OF THE DEPARTMENT OF POLYMER ENGINEERING VISION To offer quality education and training in Polymer Engineering through well structured curriculum and syllabi to produce engineers with sound technical knowledge and expertise to meet the needs of the society MISSION • To impart knowledge and skill in the field of Polymer Science and Engineering through well designed programs • To equip the students with necessary skill in the development of polymers and polymeric products using appropriate techniques and software • To promote engineering spirit for the product development through effective integration of design engineering and material technology • To undertake research in multi- disciplinary polymer science and engineering and related areas and to encourage enterprise, innovation, growth and development in the emerging areas of new technology • To develop analytical skills, leadership quality and team sprit through balanced curriculum and a judicial mix of co-curricular, extra-curricular and professional society activities • To disseminate knowledge through seminars, conference and research publications for the benefit of society B.Tech. -

Regulations 2017 Curriculum and Syllabi B.Tech. Polymer
REGULATIONS 2017 CURRICULUM AND SYLLABI B.TECH. POLYMER ENGINEERING B.Tech. Polymer Engineering Regulations 2017 B.S. Abdur Rahman Crescent Institute of Science and Technology 2 B.Tech. Polymer Engineering Regulations 2017 VISION AND MISSION OF THE INSTITUTION VISION B.S. Abdur Rahman Crescent Institute of Science and Technology aspires to be a leader in Education, Training and Research in Engineering, Science, Technology and Management and to play a vital role in the Socio-Economic progress of the Country. MISSION • To blossom into an internationally renowned Institution • To empower the youth through quality education and to provide professional leadership • To achieve excellence in all its endeavors to face global challenges • To provide excellent teaching and research ambience • To network with global institutions of Excellence, Business, Industry and Research Organizations • To contribute to the knowledge base through Scientific enquiry, Applied research and Innovation B.S. Abdur Rahman Crescent Institute of Science and Technology 3 B.Tech. Polymer Engineering Regulations 2017 B.S. Abdur Rahman Crescent Institute of Science and Technology 4 B.Tech. Polymer Engineering Regulations 2017 DEPARTMENT OF POLYMER ENGINEERING VISION AND MISSION VISION To offer quality education and training in Polymer Engineering through well structured curriculum and syllabi to produce engineers with sound technical knowledge and expertise to meet the needs of the society. MISSION To impart knowledge and skill in the field of Polymer Science and Engineering -

REGULATIONS 2017 CURRICULUM and SYLLABI B.Tech
REGULATIONS 2017 CURRICULUM AND SYLLABI B.Tech. POLYMER ENGINEERING B.Tech. Polymer Engineering Regulations 2017 B.S. Abdur Rahman Crescent Institute of Science and Technology 2 B.Tech. Polymer Engineering Regulations 2017 VISION AND MISSION OF THE INSTITUTION VISION B.S. Abdur Rahman Institute of Science and Technology aspires to be a leader in Education, Training and Research in Engineering, Science, Technology and Management and to play a vital role in the Socio-Economic progress of the Country. MISSION • To blossom into an internationally renowned University • To empower the youth through quality education and to provide professional leadership • To achieve excellence in all its endeavours to face global challenges • To provide excellent teaching and research ambience • To network with global institutions of Excellence, Business, Industry and Research Organizations • To contribute to the knowledge base through Scientific enquiry, Applied research and Innovation B.S. Abdur Rahman Crescent Institute of Science and Technology 3 B.Tech. Polymer Engineering Regulations 2017 B.S. Abdur Rahman Crescent Institute of Science and Technology 4 B.Tech. Polymer Engineering Regulations 2017 DEPARTMENT OF POLYMER ENGINEERING VISION AND MISSION VISION To offer quality education and training in Polymer Engineering through a well structured curriculum and syllabi to produce engineers with sound technical knowledge and expertise to meet the needs of the society. MISSION To impart knowledge and skill in the field of Polymer Science and Engineering through -

Introduction to Polymer Science and Technology
Mustafa Akay Introduction to Polymer Science and Technology 2 Download free eBooks at bookboon.com Introduction to Polymer Science and Technology © 2015 Mustafa Akay & bookboon.com ISBN 978-87-403-0087-1. 3 Download free eBooks at bookboon.com Introduction to Polymer Science and Technology Contents Contents Preface 8 Acknowledgements 9 1 Introduction 10 1.1 History of the development of polymers 10 1.2 Why a clear understanding of material is important? 12 1.3 What can be achieved by appropriate selection of polymer-based materials? 17 1.4 What makes polymers versatile? 20 2 Polymerisation 31 2.1 Polymerisation mechanisms 31 2.2 Polymerisation processes 36 2.3 Polymerisation reactors 39 2.4 Catalysts 42 2.5 Molecular weight and molecular weight distributions 47 2.6 Self-assessment questions 50 4 Download free eBooks at bookboon.com Introduction to Polymer Science and Technology Contents 3 Polymer processing 54 3.1 Concept of rheology 54 3.2 Processing and forming thermoplastics 56 3.3 Processing and forming thermosetting polymers 98 3.4 Self-assessment questions 109 4 Microstructure 111 4.1 Stereoregularity 112 4.2 Morphology in semi-crystalline thermoplastics 113 4.3 Degree of crystallinity 116 4.4 Crosslinking 124 4.5 Copolymer arrangements 126 4.6 Domain structures 127 4.7 Degree of molecular orientation 128 4.8 Self-assessment questions 130 5 Behaviour of polymers 133 5.1 Degradation of Polymers 133 5.2 Viscoelasticity 134 5.3 Relaxation transitions 150 5.4 Self-assessment questions 158 5 Download free eBooks at bookboon.com Introduction -

Institute of Management Nirma University Mandatory Disclosure.Pdf
INSTITUTE OF DIPLOMA STUDIES NIRMA UNIVERSITY MANDATORY DISCLOSURE Submitted to Directorate of Technical Education Gandhinagar Towards VARIATION IN THE APPROVED INTAKE CAPACITY OF DIPLOMA COURSES IN ENGINEERING AND TECHNOLOGY AND CONTINUATION OF APPROVAL FOR THE ACADEMIC YEAR 2010-11 ‐ 1 – MANDATORY DISCLOSURE MANDATORY DISCLOSURE BY INSTITUTIONS RUNNING AICTE APPROVED ENGINEERING/TECHNOLOGY/PHARMACY PROGRAMMES TO BE INCLUDED IN THEIR RESPECTIVE INFORMATION BROCHURE, DISPLAYED ON THEIR WEBSITE AND TO BE SUBMITTED TO AICTE EVERY YEAR LATEST BY 30TH APRIL TOGETHER WITH ITS URL The following information is to be given in the Information Brochure besides being hosted on the Institution’s official Website. “The information has been provided by the concerned institution and the onus of authenticity lies with the institution and not on AICTE.” 1.1 NAME OF THE INSTITUTION ¾ Address including telephone, Fax, e‐mail. Name INSTITUTE OF DIPLOMA STUDIES, NIRMA UNIVERSITY Sarkhej‐Gandhinagar Highway Village Chharodi District Ahmedabad Pin Code 382 481 State Gujarat Phone No. 02717‐ 241911‐15 Fax No. 02717‐241917 E‐Mail: [email protected] Web site www.nirmauni.ac.in/ids Nearest Rly Ahmedabad – 25 kms. Station Nearest Airport Ahmedabad – 23 kms. II. NAME & ADDRESS OF THE PRINCIPAL ¾ Address including telephone, Fax, e‐mail. Name Prof. Suresh Pareek Designation Principal (In charge) Institute of Diploma Studies Nirma University Sarkhej‐Gandhinagar Highway Ahmedabad 382 481, Gujarat Phone No. (O) 02717‐ 241911‐15 Fax No. 02717‐241917 E‐Mail: [email protected] Website: www.nirmauni.ac.in/ids ‐ 2 – III. Name OF THE AFFILIATING UNIVERSITY Name Nirma University * Address Sarkhej‐Gandhinagar Highway, Ahmedabad – 382481 Pin Code 382481 Period of A constituent Unit Affiliation STD Code 02717 Phone No. -

Background Document to the Opinions on the Annex XV Dossier Proposing Restrictions on Mercury in Measuring Devices
BACKGROUND DOCUMENT TO RAC AND SEAC OPINIONS ON MERCURY IN MEASURING DEVICES Committee for Risk Assessment (RAC) Committee for Socio-economic Analysis (SEAC) Background document to the opinions on the Annex XV dossier proposing restrictions on Mercury in measuring devices ECHA/RAC/ RES-O-0000001363-81-02/F ECHA/SEAC/ RES-O-0000001363-81-03/S1 Mercury EC number: 231-106-7 CAS number: 7439-97-6 This Background Document (BD) shall be regarded as further reference material to the opinions of the Committees for Risk Assessment and Socio-economic Analysis. It contains further details and assessment in addition/beyond the justifications provided in the opinions including, where relevant, information that has been received during the opinion making process and may be used to better understand the opinions and their justifications. The BD is a supporting document based on the Annex XV restriction report submitted by MS, and updated to support the opinions of the Committees . 15 September 2011 i Preface The existing restriction in Entry 18a of Annex XVII to the REACH Regulation on mercury in measuring devices includes a review clause. According to the clause, the Commission was to carry out a review of the availability of reliable safer alternatives that are technically and economically feasible for mercury containing measuring devices and where such alternatives are available present, if appropriate, a proposal to extent the existing restriction. The Commission sent its review report to ECHA on 20 November 2009 and requested ECHA to prepare a corresponding Annex XV restriction report. This Background Document (BD) concerns the industrial and professional uses of mercury in measuring devices as the existing entry in Annex XVII already restricts the placing on the market of mercury containing measuring devices for general public.