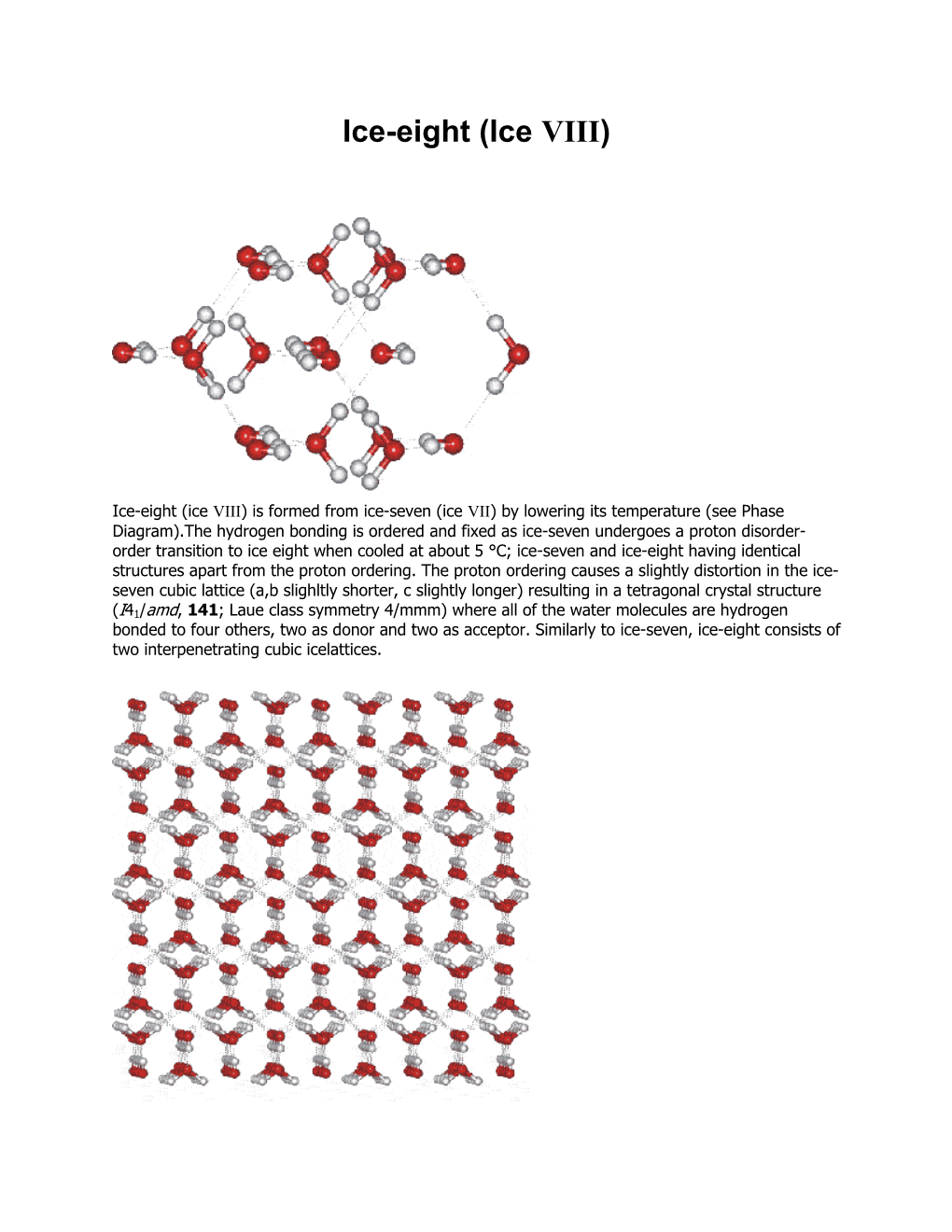
Ice-Eight (Ice VIII)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evasive Ice X and Heavy Fermion Ice XII: Facts and Fiction About High
Physica B 265 (1999) 113—120 Evasive ice X and heavy fermion ice XII: facts and fiction about high-pressure ices W.B. Holzapfel* Fachbereich Physik, Universita( t-GH Paderborn, D-33095 Paderborn, Germany Abstract Recent theoretical and experimental results on the structure and dynamics of ice in wide regions of pressure and temperature are compared with earlier models and predictions to illustrate the evasive nature of ice X, which was originally introduced as completely ordered form of ice with short single centred hydrogen bonds isostructural CuO. Due to the lack of experimental information on the proton ordering in the pressure and temperature region for the possible occurrence of ice X, effects of thermal and quantum delocalization are discussed with respect to the shape of the phase diagram and other structural models consistent with present optical and X-ray data for this region. Theoretical evidences for an additional orthorhombic modification (ice XI) at higher pressures are confronted with various reasons supporting a delocalization of the protons in the form of a heavy fermion system with very unique physical properties characterizing this fictitious new phase of ice XII. ( 1999 Elsevier Science B.V. All rights reserved. Keywords: Ices; Hydrogen bonds; Phase diagram; Equation of states 1. Introduction tunnelling [2]. Detailed Raman studies on HO and DO ice VIII to pressures in the range of The first in situ X-ray studies on HO and DO 50 GPa [3] revealed the expected softening in the ice VII under pressures up to 20 GPa [1] together molecular stretching modes and led to the deter- with a simple twin Morse potential (TMP) model mination of a critical O—H—O bond length of " for protons or deuterons in hydrogen bonds [2] R# 232 pm, where the central barrier of the effec- allowed almost 26 years ago first speculations tive double-well potential should disappear [4]. -

Novel Hydraulic Structures and Water Management in Iran: a Historical Perspective
Novel hydraulic structures and water management in Iran: A historical perspective Shahram Khora Sanizadeh Department of Water Resources Research, Water Research Institute������, Iran Summary. Iran is located in an arid, semi-arid region. Due to the unfavorable distribution of surface water, to fulfill water demands and fluctuation of yearly seasonal streams, Iranian people have tried to provide a better condition for utilization of water as a vital matter. This paper intends to acquaint the readers with some of the famous Iranian historical water monuments. Keywords. Historic – Water – Monuments – Iran – Qanat – Ab anbar – Dam. Structures hydrauliques et gestion de l’eau en Iran : une perspective historique Résumé. L’Iran est situé dans une région aride, semi-aride. La répartition défavorable des eaux de surface a conduit la population iranienne à créer de meilleures conditions d’utilisation d’une ressource aussi vitale que l’eau pour faire face à la demande et aux fluctuations des débits saisonniers annuels. Ce travail vise à faire connaître certains des monuments hydrauliques historiques parmi les plus fameux de l’Iran. Mots-clés. Historique – Eau – Monuments – Iran – Qanat – Ab anbar – Barrage. I - Introduction Iran is located in an arid, semi-arid region. Due to the unfavorable distribution of surface water, to fulfill water demands and fluctuation of yearly seasonal streams, Iranian people have tried to provide a better condition for utilization of water as a vital matter. Iran is located in the south of Asia between 44º 02´ and 63º 20´ eastern longitude and 25º 03´ to 39º 46´ northern latitude. The country covers an area of about 1.648 million km2. -

11Th International Conference on the Physics and Chemistry of Ice, PCI
11th International Conference on the Physics and Chemistry of Ice (PCI-2006) Bremerhaven, Germany, 23-28 July 2006 Abstracts _______________________________________________ Edited by Frank Wilhelms and Werner F. Kuhs Ber. Polarforsch. Meeresforsch. 549 (2007) ISSN 1618-3193 Frank Wilhelms, Alfred-Wegener-Institut für Polar- und Meeresforschung, Columbusstrasse, D-27568 Bremerhaven, Germany Werner F. Kuhs, Universität Göttingen, GZG, Abt. Kristallographie Goldschmidtstr. 1, D-37077 Göttingen, Germany Preface The 11th International Conference on the Physics and Chemistry of Ice (PCI- 2006) took place in Bremerhaven, Germany, 23-28 July 2006. It was jointly organized by the University of Göttingen and the Alfred-Wegener-Institute (AWI), the main German institution for polar research. The attendance was higher than ever with 157 scientists from 20 nations highlighting the ever increasing interest in the various frozen forms of water. As the preceding conferences PCI-2006 was organized under the auspices of an International Scientific Committee. This committee was led for many years by John W. Glen and is chaired since 2002 by Stephen H. Kirby. Professor John W. Glen was honoured during PCI-2006 for his seminal contributions to the field of ice physics and his four decades of dedicated leadership of the International Conferences on the Physics and Chemistry of Ice. The members of the International Scientific Committee preparing PCI-2006 were J.Paul Devlin, John W. Glen, Takeo Hondoh, Stephen H. Kirby, Werner F. Kuhs, Norikazu Maeno, Victor F. Petrenko, Patricia L.M. Plummer, and John S. Tse; the final program was the responsibility of Werner F. Kuhs. The oral presentations were given in the premises of the Deutsches Schiffahrtsmuseum (DSM) a few meters away from the Alfred-Wegener-Institute. -

Electromagnetism Based Atmospheric Ice Sensing Technique - a Conceptual Review
Int. Jnl. of Multiphysics Volume 6 · Number 4 · 2012 341 Electromagnetism based atmospheric ice sensing technique - A conceptual review Umair N. Mughal*, Muhammad S. Virk and M. Y. Mustafa High North Technology Center Department of Technology, Narvik University College, Narvik, Norway ABSTRACT Electromagnetic and vibrational properties of ice can be used to measure certain parameters such as ice thickness, type and icing rate. In this paper we present a review of the dielectric based measurement techniques for matter and the dielectric/spectroscopic properties of ice. Atmospheric Ice is a complex material with a variable dielectric constant, but precise calculation of this constant may form the basis for measurement of its other properties such as thickness and strength using some electromagnetic methods. Using time domain or frequency domain spectroscopic techniques, by measuring both the reflection and transmission characteristics of atmospheric ice in a particular frequency range, the desired parameters can be determined. Keywords: Atmospheric Ice, Sensor, Polar Molecule, Quantum Excitation, Spectroscopy, Dielectric 1. INTRODUCTION 1.1. ATMOSPHERIC ICING Atmospheric icing is the term used to describe the accretion of ice on structures or objects under certain conditions. This accretion can take place either due to freezing precipitation or freezing fog. It is primarily freezing fog that causes this accumulation which occurs mainly on mountaintops [16]. It depends mainly on the shape of the object, wind speed, temperature, liquid water content (amount of liquid water in a given volume of air) and droplet size distribution (conventionally known as the median volume diameter). The major effects of atmospheric icing on structure are the static ice loads, wind action on iced structure and dynamic effects. -

Hidden Force Floating Ice
Hidden force floating ice Chang Q Sun, [email protected] Nanyang Technological University, Singapore Because of the segmental specific-heat disparity of the hydrogen bond (O:H-O) and the Coulomb repulsion between oxygen ions, cooling elongates the O:H-O bond at freezing by stretching its containing angle and shortening the H-O bond with an association of larger O:H elongation, which makes ice less dense than water, allowing it to float. Ref: [1] Mpemba effect, http://arxiv.org/abs/1501.00765 [2] Hydrogen-bond relaxation dynamics: resolving mysteries of water ice. Coord. Chem. Rev., 2015. 285: 109-165. 1.1 Anomaly: floating of ice Observations shown in Figure 1 confirmed the following: 1) Ice cube is less dense than water so it floats in water [1]. 2) The mass density (T) profile oscillates over the full temperature range [2]. 3) Cooling densification proceeds in the liquid (I) and the solid (III) phase and cooling expansion occurs to the quasi-solid phase (II) and ice in the very-low temperature regime (IV) at different rates. Density transits at 277 K (for bulk), 202 K and 50 K (for droplet of 1.4 nm size). 1 b a 1.00 0.98 ) 3 IV III II I 0.96 (g/cm 0.94 0.92 0 50 100 150 200 250 300 350 400 T (K) Figure 1. (a) Low density ice cubes float in a cup of water [1] and (b) the density (T) profile of water oscillates over the full temperature range for 1.4 nm droplet obtained using Raman and FTIR [2]. -

Book of Abstracts 14Th International Conference on the Physics And
14 th International Conference on the Physics and Chemistry of Ice © Zürich Tourismus January 8–12, 2018 | Paul Scherrer Institut | Switzerland Book of Abstracts PCI 2018 January 8 – 12, 2018 ETH Zürich, Switzerland http://indico.psi.ch/event/PCI2018 Steering Committee John S. Wettlaufer, USA (chair) Masahiko Arakawa, Japan Ian Baker, USA Thorsten Bartels-Rausch, Switzerland Yoshi Furukawa, Japan Robert Gagnon, Canada Werner F. Kuhs, Germany John LoVeday, UK Valeria Molinero, USA Maurine Montagnat, France Jan Pettersson, Sweden NAP-XPS Solutions NEAR AMBIENT PRESSURE IN-SITU SURFACE ANALYSIS SYSTEMS KEY FEATURES • In-Situ Chemical Analysis up to 100 mbar • PHOIBOS 150 NAP Electron Analyser • For Use with NAP Laboratory X-ray and UV Sources or Synchrotron Beam • Cooling Stage to Investigate Ice SPECS Surface Nano Analysis GmbH T +49 30 46 78 24-0 E [email protected] H www.specs.com Swiss snow, ice and permafrost society The Swiss Snow, Ice and Permafrost Society (SIP) is a learned society with the aim of facilitating the dialog between science, the practice and the general public. The society is dedicated to the following aims: • improvement and spreading of glaciological knowledge, • facilitates contacts between glaciological experts, practitioners and society, • takes position in glaciological questions of general interest, • supports knowledge exchange and collaboration between its members, • keeps contacts to national and international societies, and supports the next generation of scientists. The Swiss Society for Snow, Ice and Permafrost is open to all persons interested in glaciology, and is a scientific society of the Swiss Academy of Sciences (SCNAT). https://naturalsciences.ch/organisations/snow_ice_permafrost Table of contents Amorphous ices and liquid states (93) ............................................................................................. -

Open Questions on the Structures of Crystalline Water Ices ✉ Thomas Loerting 1 , Violeta Fuentes-Landete1,2, Christina M
COMMENT https://doi.org/10.1038/s42004-020-00349-2 OPEN Open questions on the structures of crystalline water ices ✉ Thomas Loerting 1 , Violeta Fuentes-Landete1,2, Christina M. Tonauer 1 & Tobias M. Gasser1 1234567890():,; Water can form a vast number of topological frameworks owing to its hydrogen- bonding ability, with 19 different forms of ice experimentally confirmed at pre- sent. Here, the authors comment on open questions and possible future dis- coveries, covering negative to ultrahigh pressures. The hydrogen bond as envisioned by Linus Pauling is key to the structures of H2O ices. A hydrogen bond can be characterised as O–H⋯O, where the hydrogen atom is linked via a short covalent bond to the donor oxygen and via the longer bond to the acceptor. The one feature that makes ice structures so diverse is that four hydrogen bonds can be formed by a water molecule, although it has only three atoms. Thereby, two atoms may act as H donors, two as H acceptors. In ice, as opposed to water clusters or liquid water, all four hydrogen bonds are developed, resulting in a tetrahedral coordination around each water molecule. This local coordination motif is called the Walrafen pentamer and is found in almost all ice structures1. Hydrogen bonding at ultrahigh pressure Figure 1 summarises the phase diagram for water in a very broad pressure regime, up to pressures as high as encountered in the interior of the ice giants, Uranus and Neptune. Only at ultrahigh pressures, exceeding 50 GPa, the Walrafen pentamer breaks down. In this pressure regime, the hydrogen atom becomes centred between the two oxygen atoms. -

Supercomputing Institute for Digital Simulation and Advanced Computation
Supercomputing Institute for Digital Simulation and Advanced Computation 2006 Annual Research Report 2006 Annual Research Report of the Supercomputing Institute for Digital Simulation and Advanced Computation Visit us on the Internet: www.msi.umn.edu Supercomputing Institute for Digital Simulation and Advanced Computation University of Minnesota 599 Walter 117 Pleasant Street SE Minneapolis, Minnesota 55455 © 2006 by the Regents of the University of Minnesota. All rights reserved. This report was prepared by Supercomputing Institute researchers and staff. Editor: Tracey A. Bartlett This publication can be made available in alternative formats for people with disabilities. Direct requests to [email protected] or call (612) 625-1818. The University of Minnesota is committed to the policy that all persons shall have equal access to its programs, facilities, and employment without regard to race, color, creed, religion, national ori- gin, sex, age, marital status, disability, public assistance status, veteran status, or sexual orientation. contains a minimum of 10% postconsumer waste Table of Contents Introduction Overview . .2 Digital Technology Center . .2 Supercomputing Institute Resources and Programs Core Resources . .3 Research Laboratories Basic Sciences Computing Laboratory . .5 Computational Genetics Laboratory . .6 Digital Technology Computational Biology Laboratory . .7 Laboratory for Large-Scale Data Analysis . .8 Medicinal Chemistry/Supercomputing Institute Visualization-Workstation Laboratory . .9 Scientific Data Management Laboratory . .10 Scientific Development and Visualization Laboratory . .11 Graduate Programs Scientific Computational Graduate Program . .12 Computational Neuroscience Program . .12 Partnerships Computational Life Sciences Program . .13 Grid and BlueGene Computing Block Grant . .14 Laboratory for Computational Science and Engineering . .15 Research Scholarship Program . .16 Undergraduate Internship Program . .17 Researchers and Administration Fellows of the Institute . -

Author Index
PHYSICAL REVIEW B VOLUME 70, NUMBER 24 15 DECEMBER 2004-II Cumulative Author Index All authors of papers published in this volume are listed alphabetically. Full titles are included in each first author’s entry. For Rapid Communi- cations an R precedes the page number. The letters (C) and (BR) following the page number indicate that a paper is a Comment or a Brief Report, respectively. References with (E) are to Errata. Aarab, H. — ͑see Mulazzi, E.͒ B 70, 155206͑15͒ and D. A. Shulyatev — First-order nature of the ferromagnetic ͑ ͒ ͑ ͒ ͒ ͑ ͒ Aarts, J. — see Rusanov, A. Yu. B 70, 024510 1 phase transition in ͑La-Ca MnO3 near optimal doping. B 70, 134414 1 ͑seeYang,Z.Q.͒ B 70, 174111͑1͒ Adams, E. M. — ͑see Nachimuthu, P.͒ B 70, 100101͑R͒͑1͒ ͑see Zhang, Y. Q.͒ B 70, 132407͑BR͒͑1͒ Adams, M. A. — ͑see Knafo, W.͒ B 70, 174401͑1͒ Abalmassov, Veniamin A. and Florian Marquardt — Electron-nuclei Adams, P. W. — ͑see Young, D. P.͒ B 70, 064508͑1͒ spin relaxation through phonon-assisted hyperfine interaction in a Adell, M., L. Ilver, J. Kanski, J. Sadowski, R. Mathieu, and V. quantum dot. B 70, 075313͑15͒ Stanciu — Photoemission studies of the annealing-induced ͑ ͒ Abanov, Ar. and Andrey V. Chubukov — Spin resonance and high- modifications of Ga0.95Mn0.05As. B 70, 125204 15 frequency optical properties of the cuprates. B 70, 100504͑R͒͑1͒ Adelmann, C., B. Daudin, R. A. Oliver, G. A. D. Briggs, and R. E. Abayev, I. — ͑see Kytin, V. G.͒ B 70, 193304͑BR͒͑15͒ Rudd — Nucleation and growth of GaN/AlN quantum dots. -
Combinatorial Entropy and Phase Diagram of Partially Ordered Ice Phases Luis G
JOURNAL OF CHEMICAL PHYSICS VOLUME 121, NUMBER 20 22 NOVEMBER 2004 Combinatorial entropy and phase diagram of partially ordered ice phases Luis G. MacDowell, Eduardo Sanz, Carlos Vega, and Jose´ Luis F. Abascal Dpto. de Quı´mica Fı´sica, Facultad de Ciencias Quı´micas, Universidad Complutense, 28040 Madrid, Spain ͑Received 8 July 2004; accepted 30 August 2004͒ A close analytical estimate for the combinatorial entropy of partially ordered ice phases is presented. The expression obtained is very general, as it can be used for any ice phase obeying the Bernal-Fowler rules. The only input required is a number of crystallographic parameters, and the experimentally observed proton site occupancies. For fully disordered phases such as hexagonal ice, it recovers the result deduced by Pauling, while for fully ordered ice it is found to vanish. Although the space groups determined for ice I, VI, and VII require random proton site occupancies, it is found that such random allocation of protons does not necessarily imply random orientational disorder. The theoretical estimate for the combinatorial entropy is employed together with free energy calculations in order to obtain the phase diagram of ice from 0 to 10 GPa. Overall qualitative agreement with experiment is found for the TIP4P model of water. An accurate estimate of the combinatorial entropy is found to play an important role in determining the stability of partially ordered ice phases, such as ice III and ice V. © 2004 American Institute of Physics. ͓DOI: 10.1063/1.1808693͔ I. INTRODUCTION coexistence lines between ordered and disordered phases, or on dielectric response measurements.3 The behavior and properties of ice have provided a con- With the advent of neutron scattering technology for stant challenge to our understanding of statistical mechanics crystal structure determination, the actual hydrogen arrange- and other related areas.1–3 No other naturally abundant sub- ments could be definitively settled for the first time. -
High Density Amorphous Ice at Room Temperature
High density amorphous ice at room temperature Jing-Yin Chen and Choong-Shik Yoo1 Institute for Shock Physics and Department of Chemistry, Washington State University, Pullman, WA 99164–2816 Edited* by Russell J. Hemley, Carnegie Institution of Washington, Washington, DC, and approved March 23, 2011 (received for review January 14, 2011) The phase diagram of water is both unusual and complex, exhibit- neighbor oxygen-oxygen distance in ice VII decreases, eventually ing a wide range of polymorphs including proton-ordered or dis- bringing each of the hydrogen atoms to the midway points of ordered forms. In addition, a variety of stable and metastable the neighboring oxygen atoms (i.e., symmetrized ice-X) above – forms are observed. The richness of H2O phases attests the versa- 60 70 GPa (22). Above 150 GPa ice X further transforms to tility of hydrogen-bonded network structures that include kineti- an antifluorite-like structure (23). At higher pressures, it is also cally stable amorphous ices. Information of the amorphous solids, predicted to transform to orthorhombic structures such as Pbcm, however, is rarely available especially for the stability field and Pbca, and Cmcm (24). From 40–100 GPa, under increasing tem- transformation dynamics—but all reported to exist below the peratures, ab initio molecular dynamics (MD) simulations have crystallization temperature of approximately 150–170 K below found evidence of enhanced hydrogen self-diffusion and signifi- 4–5 GPa. Here, we present the evidence of high density amorphous cant ionic conductivity below the melting curve of H2O (25, 26). (HDA) ice formed well above the crystallization temperature at Yet, with decreasing temperatures (below 270 K), hydrogen-dis- 1 GPa—well inside the so-called “no-man’s land.” It is formed from ordered ice VII transforms to antiferroelectrically dipole-ordered metastable ice VII in the stability field of ice VI under rapid com- ice VIII (27). -

Anomalous Hydrogen Dynamics of the Ice VII–VIII Transition Revealed by High-Pressure Neutron Diffraction
Anomalous hydrogen dynamics of the ice VII–VIII transition revealed by high-pressure neutron diffraction Kazuki Komatsua,1, Stefan Klotzb, Shinichi Machidac, Asami Sano-Furukawad, Takanori Hattorid, and Hiroyuki Kagia aGeochemical Research Center, Graduate School of Science, The University of Tokyo, Bunkyo-ku, Tokyo 113-0033, Japan; bInstitut de Minéralogie, de Physique des Matériaux et de Cosmochimie, CNRS UMR 7590, Sorbonne Université, F-75252 Paris, France; cNeutron Science and Technology Center, CROSS, Tokai, Naka, Ibaraki 319-1106, Japan; and dJ-PARC Center, Japan Atomic Energy Agency, Tokai, Naka, Ibaraki 319-1195, Japan Edited by Pablo G. Debenedetti, Princeton University, Princeton, NJ, and approved February 18, 2020 (received for review November 20, 2019) Above 2 GPa the phase diagram of water simplifies considerably boundary, and collected diffraction patterns of the obtained and exhibits only two solid phases up to 60 GPa, ice VII and ice VIII. material at 93 K (Fig. 1). Experimental details are described in The two phases are related to each other by hydrogen ordering, Methods, and the details of temperature change in cooling are given with the oxygen sublattice being essentially the same. Here we in SI Appendix,Fig.S1. We find indeed highly H-disordered phase present neutron diffraction data to 15 GPa which reveal that the VIII at around 10 GPa, but not for pressures significantly below and rate of hydrogen ordering at the ice VII–VIII transition decreases above. Our results are remarkable since they allow a direct link with strongly with pressure to reach timescales of minutes at 10 GPa. recent high-pressure electric conductivity measurements: The low- Surprisingly, the ordering process becomes more rapid again upon frequency limit (corresponding to direct current [dc]) of elec- further compression.