The Uveoscleral Outflow Routes
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Glaucoma Is One of the Leading Causes of Blindness in Animals and People
www.southpaws.com SouthPaws Ophthalmology Service 8500 Arlington Boulevard Fairfax, Virginia 22031 Tel: 703.752.9100 Fax: 703.752.9200 Glaucoma is one of the leading causes of blindness in animals and people. Glaucoma is defined as increased pressure within the eye (greater than 25 mmHg), beyond that which is compatible with normal ocular function and vision. It is caused by a disturbance in the flow of fluid within and out of the globe. Primary glaucoma occurs when there are no other underlying problems causing the pressure elevation and is usually inherited. Secondary glaucoma occurs when there are other problems within the eye which have contributed to the elevated pressure. Examples of these underlying problems include uveitis (inflammation) and luxation (shifting from the normal position) of the lens. Glaucoma The fluid that fills the eye (the aqueous humour) is produced by the ciliary body, a structure which is located behind the iris and circles for 360 degrees around the eye. The fluid produced by the ciliary body flows through the pupil and fills the anterior chamber. Normally, this fluid flows out from the eye through the iridocorneal drainage angle, a structure encircling the eye where the iris and cornea meet. In a normal eye the fluid production and outflow are evenly matched, and this keeps the intraocular pressure steady. Glaucoma occurs when there is an obstruction to the outflow of the fluid which causes the fluid to build up and increase the intraocular pressure. As the pressure increases, several changes can occur within the eye: 1) Increased pressure forces fluid into the cornea and disrupts the arrangement of the protein fibers that compose the cornea. -

Basic Anatomy & Physiology Instruction Manual
B>ÈV A˜>Ìœ“Þ >˜` *…ÞÈœ•IA˜ ˆ˜ÌÀœ Basic Anatomy & Physiology Li Ài«Àœ`ÕVi`] >`>«Ìi` ˜œÀ ÌÀ>˜ÃviÀÀi` Ìœ >˜œÌ…iÀ «>ÀÌÞ œr «>À̈ià ܈̅œÕÌ «ÀˆœÀ ÜÀˆÌÌi˜ «iÀ“ˆÃÈœ˜ œv Instruction Manual the Halth & Social Care Information Centre. An introduction for clinical coders For more information please Clinical Classifications Service contact ([email protected]) Website www.systems.hscic.gov.uk/data/clinicalcoding Reference number 3811 © Health and Social Care Information Centre Date of issue August 2014 Anatomical illustrations © Health and Social Care Information Centre and Peak Dean Associates. They may not be reproduced, adapted nor transferred to another party or parties without written permission of Health and Social Care Information Centre. 1 Foreword This manual is intended to be used as a basic instruction tool, rather than a comprehensive course of study or a reference book. It will help promote an understanding of diseases and operations described in casenotes by providing information on body systems, how they work together, and the terminology used to describe them. The intended result is that those responsible for clinical coding will be better able to translate these concepts into the appropriate clinical codes. Contents Section 1..................................................................................................................................................................................................................................................... 5 Introduction to Medical Terminology, Anatomy -

The Effects of Sensory Denervation on the Responses of the Rabbit Eye to Prostaglandin E1, Bradykinin and Substance P J.M
Br. J. Pharmac. (1980), 69, 495-502 THE EFFECTS OF SENSORY DENERVATION ON THE RESPONSES OF THE RABBIT EYE TO PROSTAGLANDIN E1, BRADYKININ AND SUBSTANCE P J.M. BUTLER & B.R. HAMMOND Department of Experimental Ophthalmology, Institute of Ophthalmology, Judd Street, London, WCIH 9QS 1 Six to eight days after diathermic destruction of the fifth cranial nerve in the rabbit, the ocular hypertensive and miotic responses to intracameral administration of capsaicin, bradykinin, and prostaglandin E1 were greatly reduced or completely abolished. The response to substance P was not abolished. 2 A response could still be obtained to chemical irritants 36 h after coagulation of the nerve and it is deduced that manifestation of the response is dependent upon functional sensory nerve terminals, and is independent of central connections. 3 It is suggested that prostaglandin E1 and bradykinin act directly upon the sensory nerve endings and that propagation of the response is augmented by axon reflex. 4 In view of the ability of substance P to induce miosis in the denervated eyes, it is presumed that its actions are not mediated via sensory nerves. 5 It is considered possible that the mediator(s) released from sensory nerve endings after chemical irritation or antidromic stimulation may act in the same way as substance P with regard to the miotic effect. 6 Synthetic substance P will only produce ocular hypertension in doses which induce a maximal miotic response. This may either be a question of access or a partial resemblance to the endogenous mediator. Introduction The anterior segment of the rabbit eye responds to nitrogen mustard were reduced after retrobulbar an- mechanical or chemical irritation by miosis, vasodila- aesthesia and abolished after postganglionic division tation and a breakdown of the blood-aqueous barrier. -

The Effect of Topical Application of 0.15% Ganciclovir Gel on Cytomegalovirus Corneal Endotheliitis
Clinical science The effect of topical application of 0.15% ganciclovir gel on cytomegalovirus corneal endotheliitis Noriko Koizumi,1,2 Dai Miyazaki,3 Tomoyuki Inoue,4 Fumie Ohtani,3 Michiko Kandori-Inoue,3 Tsutomu Inatomi,1 Chie Sotozono,1 Hiroko Nakagawa,1 Tomoko Horikiri,1 Mayumi Ueta,5 Takahiro Nakamura,5 Yoshitsugu Inoue,3 Yuichi Ohashi,4 Shigeru Kinoshita5 ▸ Additional material is ABSTRACT immunocompetent individuals suffering from anter- published online only. To view Background/aims The aim of this study was to ior uveitis with ocular hypertension45and corneal please visit the journal online – evaluate the therapeutic efficacy and drug transfer of endotheliitis.368 Clinical evidence of CMV-related (http://dx.doi.org/10.1136/ 6–11 bjophthalmol-2015-308238). topical application of 0.15% ganciclovir (GCV) gel on endotheliitis has accumulated, and current 1 cytomegalovirus (CMV) corneal endotheliitis. emphasis is on the importance of early diagnosis Department of 12–14 Ophthalmology, Kyoto Methods This study is a multicentre, prospective, and treatment. Prefectural University of interventional case series. Seven eyes of seven Several studies, including ours, have reported the Medicine, Kyoto, Japan immunocompetent patients diagnosed with CMV corneal usefulness of the systemic and topical application 2 Department of Biomedical endotheliitis, based on clinical manifestations and of anti-CMV medications, such as GCV and valgan- Engineering, Faculty of Life and 36810–14 Medical Sciences, Doshisha qualitative PCR, were enrolled in this study. The patients ciclovir. Our recent study of 106 eyes University, Kyoto, Japan were treated with topical applications of 0.15% GCV gel with CMV endotheliitis in Japan involved the sys- 3Division of Ophthalmology six times daily for 12 weeks without concomitant temic administration of anti-CMV drugs in 67.9% and Visual Science, Faculty of systemic GCV. -
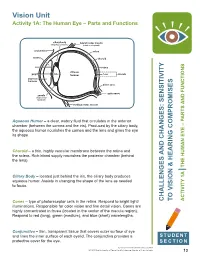
Vision Unit Activity 1A: the Human Eye – Parts and Functions
Vision Unit Activity 1A: The Human Eye – Parts and Functions ciliary body lateral rectus muscle (ring-shaped muscle) (rotation of eyeball) conjunctiva sclera cornea choroid iris retina vitreous pupil lens humour fovea macula aqueous humour blind spot optic nerve zonula (suspensory ligament) medical rectus muscle Aqueous Humor – a clear, watery fluid that circulates in the anterior chamber (between the cornea and the iris). Produced by the ciliary body, the aqueous humor nourishes the cornea and the lens and gives the eye its shape. Choroid – a thin, highly vascular membrane between the retina and the sclera. Rich blood supply nourishes the posterior chamber (behind the lens). Ciliary Body – located just behind the iris, the ciliary body produces aqueous humor. Assists in changing the shape of the lens as needed to focus. Cones – type of photoreceptor cells in the retina. Respond to bright light/ AND CHANGES: SENSITIVITY CHALLENGES VISION & HEARING COMPROMISES TO AND FUNCTIONS THE HUMAN EYE – PARTS 1A ACTIVITY illuminations. Responsible for color vision and fine detail vision. Cones are highly concentrated in fovea (located in the center of the macula region). Respond to red (long), green (medium), and blue (short) wavelengths. Conjunctiva – thin, transparent tissue that covers outer surface of eye and lines the inner surface of each eyelid. The conjunctiva provides a STUDENT protective cover for the eye. SECTION Teacher Enrichment Initiatives/CAINE 2013 © The University of Texas Health Science Center at San Antonio 13 Eyelid – Moveable fold of skin over the eye with lashes and glands along its margin. Eyelids provide protection from the environment, injury, and light. -

Postmortem Analyses of Vitreous Fluid
From the Department of Oncology-Pathology Karolinska Institutet, Stockholm, Sweden POSTMORTEM ANALYSES OF VITREOUS FLUID Brita Zilg Stockholm 2015 All previously published papers were reproduced with permission from the publisher. Published by Karolinska Institutet. Cover picture: Anatomy of the eye, by Ibn al-Haytham, ~1000 A.D. Printed by AJ E-Print 2015. © Brita Zilg, 2015 ISBN 978-91-7676-104-5 POSTMORTEM ANALYSES OF VITREOUS FLUID THESIS FOR DOCTORAL DEGREE (Ph.D.) By Brita Zilg Principal Supervisor: Opponent: Prof. Henrik Druid Prof. Burkhard Madea Karolinska Institutet University of Bonn Department of Oncology-Pathology Institute of Forensic Medicine Co-supervisor: Examination Board: Assoc. prof. Sören Berg Assoc. prof. Anders Ottosson University of Linköping University of Lund Department of Medicine and Health Department of Clinical Sciences Assoc. prof. Erik Edston University of Linköping Department of Medicine and Health Assoc. prof. Bo-Michael Bellander Karolinska Institutet Department of Clinical Neuroscience Institutionen för Onkologi-Patologi Postmortem Analyses of Vitreous Fluid AKADEMISK AVHANDLING som för avläggande av medicine doktorsexamen vid Karolinska Institutet offentligen försvaras i föreläsningssalen Rockefeller Fredagen den 6 november 2015, kl 09.00 av Brita Zilg Principal Supervisor: Opponent: Prof. Henrik Druid Prof. Burkhard Madea Karolinska Institutet University of Bonn Department of Oncology-Pathology Institute of Forensic Medicine Co-supervisor: Examination Board: Assoc. prof. Sören Berg Assoc. prof. Anders Ottosson University of Linköping University of Lund Department of Medicine and Health Department of Clinical Sciences Assoc. prof. Erik Edston University of Linköping Department of Medicine and Health Assoc. prof. Bo-Michael Bellander Karolinska Institutet Department of Clinical Neuroscience ABSTRACT The identification of various various medical conditions postmortem is often difficult. -

Chapter 1 Anatomy
LN_C01.qxd 7/19/07 14:37 Page 1 Chapter 1 Anatomy Learning objectives To learn the anatomy of the eye, orbit and the third, fourth and sixth cranial nerves, to permit an understanding of medical conditions affecting these structures. Introduction A knowledge of ocular anatomy and function is important to the understanding of eye diseases. A brief outline is given below. Gross anatomy The eye (Fig. 1.1) comprises: l A tough outer coat which is transparent anteriorly (the cornea) and opaque pos- teriorly (the sclera). The junction between the two is called the limbus. The extra- ocular muscles attach to the outer sclera while the optic nerve leaves the globe posteriorly. l A rich vascular coat (the uvea) forms the choroid posteriorly, which is lined by and firmly attached to the retina. The choroid nourishes the outer two-thirds of the retina. Anteriorly, the uvea forms the ciliary body and the iris. l The ciliary body contains the smooth ciliary muscle, whose contraction allows lens shape to alter and the focus of the eye to be changed. The ciliary epithelium secretes aqueous humour and maintains the ocular pressure. The ciliary body pro- vides attachment for the iris, which forms the pupillary diaphragm. 1 LN_C01.qxd 7/19/07 14:37 Page 2 Chapter 1 Anatomy Cornea Anterior chamber Schlemm's canal Limbus Iridocorneal angle Iris Conjunctiva Zonule Posterior chamber Lens Ciliary body Uvea Ora serrata Tendon of Choroid extraocular muscle Sclera Retina Vitreous Cribriform plate Optic nerve Fovea Figure 1.1 The basic anatomy of the eye. l The lens lies behind the iris and is supported by fine fibrils (the zonule) running under tension between the lens and the ciliary body. -

AQUEOUS HUMOR DYNAMICS and the PATHOGENESIS of GLAUCOMA in ANIMALS Paul E. Miller, DVM, Diplomate ACVO Clinical Professor Of
AQUEOUS HUMOR DYNAMICS AND THE PATHOGENESIS OF GLAUCOMA IN ANIMALS Paul E. Miller, DVM, Diplomate ACVO Clinical Professor of Comparative Ophthalmology, School of Veterinary Medicine University of Wisconsin-Madison, USA. I. Aqueous Humor A. Composition: Aqueous is a transparent, colorless solution continuously formed from plasma by the epithelial cells of the ciliary processes. It composition and formation resembles cerebrospinal fluid. Notable features of its composition include: 1. Very low protein concentration (0.5% of plasma). Proteins found in normal eyes include Albumin, IgG (but not IgA, IgM and IgD as these are larger molecules) and trace quantities of complement, and the fibrinolytic and coagulation system. Plasminogen and plasminogen proactivators are in more substantive concentrations. There is very low concentrations of inhibitors of plasminogen activators) to ensure that the trabecular meshwork remains free of fibrin. 2. Most species have very high concentrations of ascorbate in the aqueous which is actively secreted into the posterior chamber. Ascorbate is concentrated by the lens epithelium and has been shown to have a protective effect against UV-induced DNA damage to lens epithelium. Ascorbate may function as an antioxidant, regulate the sol–gel balance of mucopolysaccharides in the trabecular meshwork, or partially absorb UV radiation. Diurnal mammals have approximately 35 times the concentration of aqueous ascorbate of nocturnal mammals. 3. Lactate is produced as a result of the glycolytic degradation of glucose by both the ciliary body and the retina. It diffuses into the posterior chamber but is present at only marginally higher concentration than in the plasma at this site. However, it accumulates in the anterior chamber at considerably higher concentration than in the plasma presumably due to contributions by the lens, iris and posterior cornea. -

Distribution of Ascorbate in the Anterior Bovine Eye
Distribution of Ascorbate in the Anterior Bovine Eye Amund Ringvold, Erlend Anderssen, and Inge Kjønniksen PURPOSE. To analyze the ascorbate distribution in the anterior eye wall to better understand the functional significance of this compound in the eye. METHODS. Ascorbic acid was determined by high-performance liquid chromatography using an LC-10 system (Shimadzu, Kyoto, Japan). Bovine eye samples were used. RESULTS. The highest ascorbate concentration was observed in the corneal epithelium, with significantly higher values in the central (1.56 mg/g) than in the peripheral (1.39 mg/g) area. The ascorbate content was similar in the corneal stroma (0.22 mg/g), the Descemet’s membrane (DM)/endothelium (0.22 mg/g), and the aqueous humor (0.21 mg/ml). By comparison, the sclera (0.15 mg/g) and the conjunctiva (0.11 mg/g) showed lower values, as did the lacrimal gland (0.09 mg/g) and the serum (0.0008 mg/ml). CONCLUSIONS. (1) Peak ascorbate concentration was observed in the central corneal epithelium covering the pupillary area. This is compatible with the idea that the ascorbate may act as an UV filter shielding internal eye structures from radiation damage. (2) The ascorbate concentration in the corneal stroma and DM/endothelium was as high as in the aqueous humor, and it is suggested that the aqueous humor plays a key role in the distribution of ascorbate to the anterior eye wall. (Invest Ophthalmol Vis Sci. 2000;41:20–23) ince the first report of significant amounts of ascorbate in better understanding of the ascorbate distribution in the cor- the aqueous humor,1 high concentrations have been ob- nea and its surrounding tissues may provide some clues as to its served in many parts of the eye,2 with peak values in the functional aspects, so we decided to analyze the ascorbate S 3,4 corneal epithelium. -

VU Research Portal
VU Research Portal Donor tissue for corneal transplantation Rijneveld, W.J. 2010 document version Publisher's PDF, also known as Version of record Link to publication in VU Research Portal citation for published version (APA) Rijneveld, W. J. (2010). Donor tissue for corneal transplantation: trust and control. General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. E-mail address: [email protected] Download date: 02. Oct. 2021 Chapter 2 Introduction, review of the literature asdd Corneal structure 2.1 Corneal structure 2.1 Figure 1: The cornea; profile. Figure 2: Macroscopic anatomy of the human eye. Macroscopic anatomy The cornea (figure 1 and 2) is elliptical when viewed anteriorly although it is circular when viewed posteriorly, with an average of 11.7 mm. The elliptical shape is caused by an extension of the sclera superiorly and inferiorly.1 The average radius of the curvature of the central anterior corneal surface is 7.8 mm while the average radius of curvature of the sclera is 11.5 mm. -

Unconventional Aqueous Humor Outflow: a Review
Experimental Eye Research 158 (2017) 94e111 Contents lists available at ScienceDirect Experimental Eye Research journal homepage: www.elsevier.com/locate/yexer Review Unconventional aqueous humor outflow: A review * Mark Johnson a, b, c, , Jay W. McLaren d, Darryl R. Overby e a Department of Biomedical Engineering, Northwestern University, Evanston, IL, USA b Department of Mechanical Engineering, Northwestern University, Evanston, IL, USA c Department of Ophthalmology, Northwestern University, Chicago, IL, USA d Department of Ophthalmology, Mayo Clinic, Rochester, MN, USA e Department of Bioengineering, Imperial College London, London, England, UK article info abstract Article history: Aqueous humor flows out of the eye primarily through the conventional outflow pathway that includes Received 17 September 2015 the trabecular meshwork and Schlemm's canal. However, a fraction of aqueous humor passes through an Received in revised form alternative or ‘unconventional’ route that includes the ciliary muscle, supraciliary and suprachoroidal 4 January 2016 spaces. From there, unconventional outflow may drain through two pathways: a uveoscleral pathway Accepted in revised form 26 January 2016 where aqueous drains across the sclera to be resorbed by orbital vessels, and a uveovortex pathway Available online 2 February 2016 where aqueous humor enters the choroid to drain through the vortex veins. We review the anatomy, physiology and pharmacology of these pathways. We also discuss methods to determine unconventional Keywords: fl fl Aqueous humor dynamics out ow rate, including direct techniques that use radioactive or uorescent tracers recovered from tis- fl Trabecular sues in the unconventional pathway and indirect methods that estimate unconventional out ow based Uveoscleral on total outflow over a range of pressures. -

The Constitutive Proteome of Human Aqueous Humor and Race Specific
proteomes Article The Constitutive Proteome of Human Aqueous Humor and Race Specific Alterations Sai Karthik Kodeboyina 1, Tae Jin Lee 1, Lara Churchwell 1, Lane Ulrich 2, Kathryn Bollinger 2, David Bogorad 2, Amy Estes 2, Wenbo Zhi 1, Shruti Sharma 1,2 and Ashok Sharma 1,2,3,* 1 Center for Biotechnology and Genomic Medicine, Augusta University, Augusta, GA 30912, USA; [email protected] (S.K.K.); [email protected] (T.J.L.); [email protected] (L.C.); [email protected] (W.Z.); [email protected] (S.S.) 2 Department of Ophthalmology, Augusta University, Augusta, GA 30912, USA; [email protected] (L.U.); [email protected] (K.B.); [email protected] (D.B.); [email protected] (A.E.) 3 Department of Population Health Sciences, Augusta University, Augusta, GA 30912, USA * Correspondence: [email protected] Received: 15 October 2020; Accepted: 13 November 2020; Published: 18 November 2020 Abstract: Aqueous humor (AH) is the fluid in the anterior and posterior chambers of the eye that contains proteins regulating ocular homeostasis. Analysis of aqueous humor proteome is challenging, mainly due to low sample volume and protein concentration. In this study, by utilizing state of the art technology, we performed Liquid-Chromatography Mass spectrometry (LC-MS/MS) analysis of 88 aqueous humor samples from subjects undergoing cataract surgery. A total of 2263 unique proteins were identified, which were sub-divided into four categories that were based on their detection in the number of samples: High (n = 152), Medium (n = 91), Low (n = 128), and Rare (n = 1892). A total of 243 proteins detected in at least 50% of the samples were considered as the constitutive proteome of human aqueous humor.