Mathematical Models of Fluid Motion in the Vitreous Chamber of the Human
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Openoptix NCLE Study Guide V0.2
OpenOptix NCLE Study Guide Ver. 0.2 This document is licensed under the Creative Commons Attribution 3.0 License. 6/15/2009 1 About This Document The OpenOptix NCLE Study Guide, sponsored by Laramy-K Optical has been written and is maintained by volunteer members of the optical community. This document is completely free to use, share, and distribute. For the latest version please, visit www.openoptix.org or www.laramyk.com. The quality, value, and success of this document are dependent upon your participation. If you benefit from this document, we only ask that you consider doing one or both of the following: 1. Make an effort to share this document with others whom you believe may benefit from its content. 2. Make a knowledge contribution to improve the quality of this document. Examples of knowledge contributions include original (non-copyrighted) written chapters, sections, corrections, clarifications, images, photographs, diagrams, or simple suggestions. With your help, this document will only continue to improve over time. The OpenOptix NCLE Study Guide is a product of the OpenOptix initiative. Taking a cue from the MIT OpenCourseWare initiative and similar programs from other educational institutions, OpenOptix is an initiative to encourage, develop, and host free and open optical education to improve optical care worldwide. By providing free and open access to optical education the goals of the OpenOptix initiative are to: • Improve optical care worldwide by providing free and open access to optical training materials, particularly for parts of the world where training materials and trained professionals may be limited. • Provide opportunities for optical professionals of all skill levels to review and improve their knowledge, allowing them to better serve their customers and patients • Provide staff training material for managers and practitioners • Encourage ABO certification and advanced education for opticians in the U.S. -

Shape of the Posterior Vitreous Chamber in Human Emmetropia and Myopia
City Research Online City, University of London Institutional Repository Citation: Gilmartin, B., Nagra, M. and Logan, N. S. (2013). Shape of the posterior vitreous chamber in human emmetropia and myopia. Investigative Ophthalmology and Visual Science, 54(12), pp. 7240-7251. doi: 10.1167/iovs.13-12920 This is the published version of the paper. This version of the publication may differ from the final published version. Permanent repository link: https://openaccess.city.ac.uk/id/eprint/14183/ Link to published version: http://dx.doi.org/10.1167/iovs.13-12920 Copyright: City Research Online aims to make research outputs of City, University of London available to a wider audience. Copyright and Moral Rights remain with the author(s) and/or copyright holders. URLs from City Research Online may be freely distributed and linked to. Reuse: Copies of full items can be used for personal research or study, educational, or not-for-profit purposes without prior permission or charge. Provided that the authors, title and full bibliographic details are credited, a hyperlink and/or URL is given for the original metadata page and the content is not changed in any way. City Research Online: http://openaccess.city.ac.uk/ [email protected] Visual Psychophysics and Physiological Optics Shape of the Posterior Vitreous Chamber in Human Emmetropia and Myopia Bernard Gilmartin, Manbir Nagra, and Nicola S. Logan School of Life and Health Sciences, Aston University, Birmingham, United Kingdom Correspondence: Bernard Gilmartin, PURPOSE. To compare posterior vitreous chamber shape in myopia to that in emmetropia. School of Life and Health Sciences, Aston University, Birmingham, UK, METHODS. -

Glaucoma Is One of the Leading Causes of Blindness in Animals and People
www.southpaws.com SouthPaws Ophthalmology Service 8500 Arlington Boulevard Fairfax, Virginia 22031 Tel: 703.752.9100 Fax: 703.752.9200 Glaucoma is one of the leading causes of blindness in animals and people. Glaucoma is defined as increased pressure within the eye (greater than 25 mmHg), beyond that which is compatible with normal ocular function and vision. It is caused by a disturbance in the flow of fluid within and out of the globe. Primary glaucoma occurs when there are no other underlying problems causing the pressure elevation and is usually inherited. Secondary glaucoma occurs when there are other problems within the eye which have contributed to the elevated pressure. Examples of these underlying problems include uveitis (inflammation) and luxation (shifting from the normal position) of the lens. Glaucoma The fluid that fills the eye (the aqueous humour) is produced by the ciliary body, a structure which is located behind the iris and circles for 360 degrees around the eye. The fluid produced by the ciliary body flows through the pupil and fills the anterior chamber. Normally, this fluid flows out from the eye through the iridocorneal drainage angle, a structure encircling the eye where the iris and cornea meet. In a normal eye the fluid production and outflow are evenly matched, and this keeps the intraocular pressure steady. Glaucoma occurs when there is an obstruction to the outflow of the fluid which causes the fluid to build up and increase the intraocular pressure. As the pressure increases, several changes can occur within the eye: 1) Increased pressure forces fluid into the cornea and disrupts the arrangement of the protein fibers that compose the cornea. -

Ophthalmology Ophthalmology 160.01
Introduction to Ophthalmology Ophthalmology 160.01 Fall 2019 Tuesdays 12:10-1 pm Location: Library, Room CL220&223 University of California, San Francisco WELCOME OBJECTIVES This is a 1-unit elective designed to provide 1st and 2nd year medical students with - General understanding of eye anatomy - Knowledge of the basic components of the eye exam - Recognition of various pathological processes that impact vision - Appreciation of the clinical and surgical duties of an ophthalmologist INFORMATION This elective is composed of 11 lunchtime didactic sessions. There is no required reading, but in this packet you will find some background information on topics covered in the lectures. You also have access to Vaughan & Asbury's General Ophthalmology online through the UCSF library. AGENDA 9/10 Introduction to Ophthalmology Neeti Parikh, MD CL220&223 9/17 Oculoplastics Robert Kersten, MD CL220&223 9/24 Ocular Effects of Systemic Processes Gerami Seitzman, MD CL220&223 10/01 Refractive Surgery Stephen McLeod, MD CL220&223 10/08 Comprehensive Ophthalmology Saras Ramanathan, MD CL220&223 10/15 BREAK- AAO 10/22 The Role of the Microbiome in Eye Disease Bryan Winn, MD CL220&223 10/29 Retinal imaging in patients with hereditary retinal degenerations Jacque Duncan, MD CL220&223 11/05 Pediatric Ophthalmology Maanasa Indaram, MD CL220&223 11/12 Understanding Glaucoma from a Retina Circuit Perspective Yvonne Ou, MD CL220&223 11/19 11/26 Break - Thanksgiving 12/03 Retina/Innovation/Research Daniel Schwartz, MD CL220&223 CONTACT Course Director Course Coordinator Dr. Neeti Parikh Shelle Libberton [email protected] [email protected] ATTENDANCE Two absences are permitted. -

Participation of Retinal Glucagonergic Amacrine Cells in the Regulation of Eye Growth and Refractive Error: Evidence from Neurotoxins and in Vivo Immunolesioning
Participation of retinal glucagonergic amacrine cells in the regulation of eye growth and refractive error: evidence from neurotoxins and in vivo immunolesioning by Diane Rachel Nava A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Vision Science in the Graduate Division Of the University of California, Berkeley Committee in charge: Professor Christine F. Wildsoet, Chair Professor John Flanagan Professor Joseph Napoli Spring 2016 Participation of retinal glucagonergic amacrine cells in the regulation of eye growth and refractive error: evidence from neurotoxins and in vivo immunolesioning C 2016 By Diane Rachel Nava University of California, Berkeley Abstract Participation of retinal glucagonergic amacrine cells in the regulation of eye growth and refractive error: evidence from neurotoxins and in vivo immunolesioning by Diane Rachel Nava Doctor of Philosophy in Vision Science University of California, Berkeley Professor Christine Wildsoet, Chair Growth is one of the fundamental characteristics of biological systems. The study of eye growth regulation presents an interesting window that allows for the investigation of the role of the visual environment on internal processes. We now know that there is an intricate circuitry within the eye, independent of higher brain processes, that controls the growth of the eye but more needs to be elucidated about these local regulatory circuits. An improved understanding of this circuitry is critical to developing new therapies for abnormalities in eye growth regulation such as myopia, which is impacting more and more individuals around the world each day and in its more severe from, is linked to potentially blinding ocular complications. -

Basic Anatomy & Physiology Instruction Manual
B>ÈV A˜>Ìœ“Þ >˜` *…ÞÈœ•IA˜ ˆ˜ÌÀœ Basic Anatomy & Physiology Li Ài«Àœ`ÕVi`] >`>«Ìi` ˜œÀ ÌÀ>˜ÃviÀÀi` Ìœ >˜œÌ…iÀ «>ÀÌÞ œr «>À̈ià ܈̅œÕÌ «ÀˆœÀ ÜÀˆÌÌi˜ «iÀ“ˆÃÈœ˜ œv Instruction Manual the Halth & Social Care Information Centre. An introduction for clinical coders For more information please Clinical Classifications Service contact ([email protected]) Website www.systems.hscic.gov.uk/data/clinicalcoding Reference number 3811 © Health and Social Care Information Centre Date of issue August 2014 Anatomical illustrations © Health and Social Care Information Centre and Peak Dean Associates. They may not be reproduced, adapted nor transferred to another party or parties without written permission of Health and Social Care Information Centre. 1 Foreword This manual is intended to be used as a basic instruction tool, rather than a comprehensive course of study or a reference book. It will help promote an understanding of diseases and operations described in casenotes by providing information on body systems, how they work together, and the terminology used to describe them. The intended result is that those responsible for clinical coding will be better able to translate these concepts into the appropriate clinical codes. Contents Section 1..................................................................................................................................................................................................................................................... 5 Introduction to Medical Terminology, Anatomy -
How Place of Pressurization Effects Ocular Structures
HOW PLACE OF PRESSURIZATION EFFECTS OCULAR STRUCTURES Mikayla Ferchaw, Ning-Jiun Jan, Ian Sigal, PhD. Laboratory of Ocular Biomechanics, Department of Ophthalmology, University of Pittsburgh School of Medicine INTRODUCTION loading could be observed and clearly indicated on the obtained Glaucoma is the second leading cause of irreversible images produced. Once the eyes were fixed, they were blindness worldwide [1]. The main risk factor for glaucoma is cryosectioned axially. Cryosectioning is a process where the elevated intraocular pressure (IOP), which is regulated by the eyes are frozen and then sliced into very thin sections, which in production and drainage of aqueous humor in the anterior this case is 30 microns. Next, the sections were imaged with chamber of the eye [2]. Whole eye pressurization experiments polarization light microscopy and then loaded into FIJI, an can be used to understand how increased IOP affects different image processing package, where the collagen fiber bundles structures in the eye and how that results in risk for were then marked in small increments. Simultaneously, the glaucomatous damage [3]. Current pressurization experiments, original images were processed to determine collagen fiber however, consider pressurization through the anterior and orientation. The final step to this experiment was performing vitreous chamber as interchangeable and equivalent. IOP is the statistical analysis in R, a statistical coding software, to find regulated by the dynamics of the anterior chamber, not the the difference in collagen waviness in the different regions of vitreous chamber [3]. The anterior chamber is continuously the eye when pressurized through the anterior chamber versus replenished with aqueous humor via the trabecular meshwork the vitreous chamber. -

The Nervous System: General and Special Senses
18 The Nervous System: General and Special Senses PowerPoint® Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska © 2012 Pearson Education, Inc. Introduction • Sensory information arrives at the CNS • Information is “picked up” by sensory receptors • Sensory receptors are the interface between the nervous system and the internal and external environment • General senses • Refers to temperature, pain, touch, pressure, vibration, and proprioception • Special senses • Refers to smell, taste, balance, hearing, and vision © 2012 Pearson Education, Inc. Receptors • Receptors and Receptive Fields • Free nerve endings are the simplest receptors • These respond to a variety of stimuli • Receptors of the retina (for example) are very specific and only respond to light • Receptive fields • Large receptive fields have receptors spread far apart, which makes it difficult to localize a stimulus • Small receptive fields have receptors close together, which makes it easy to localize a stimulus. © 2012 Pearson Education, Inc. Figure 18.1 Receptors and Receptive Fields Receptive Receptive field 1 field 2 Receptive fields © 2012 Pearson Education, Inc. Receptors • Interpretation of Sensory Information • Information is relayed from the receptor to a specific neuron in the CNS • The connection between a receptor and a neuron is called a labeled line • Each labeled line transmits its own specific sensation © 2012 Pearson Education, Inc. Interpretation of Sensory Information • Classification of Receptors • Tonic receptors -

The Effects of Sensory Denervation on the Responses of the Rabbit Eye to Prostaglandin E1, Bradykinin and Substance P J.M
Br. J. Pharmac. (1980), 69, 495-502 THE EFFECTS OF SENSORY DENERVATION ON THE RESPONSES OF THE RABBIT EYE TO PROSTAGLANDIN E1, BRADYKININ AND SUBSTANCE P J.M. BUTLER & B.R. HAMMOND Department of Experimental Ophthalmology, Institute of Ophthalmology, Judd Street, London, WCIH 9QS 1 Six to eight days after diathermic destruction of the fifth cranial nerve in the rabbit, the ocular hypertensive and miotic responses to intracameral administration of capsaicin, bradykinin, and prostaglandin E1 were greatly reduced or completely abolished. The response to substance P was not abolished. 2 A response could still be obtained to chemical irritants 36 h after coagulation of the nerve and it is deduced that manifestation of the response is dependent upon functional sensory nerve terminals, and is independent of central connections. 3 It is suggested that prostaglandin E1 and bradykinin act directly upon the sensory nerve endings and that propagation of the response is augmented by axon reflex. 4 In view of the ability of substance P to induce miosis in the denervated eyes, it is presumed that its actions are not mediated via sensory nerves. 5 It is considered possible that the mediator(s) released from sensory nerve endings after chemical irritation or antidromic stimulation may act in the same way as substance P with regard to the miotic effect. 6 Synthetic substance P will only produce ocular hypertension in doses which induce a maximal miotic response. This may either be a question of access or a partial resemblance to the endogenous mediator. Introduction The anterior segment of the rabbit eye responds to nitrogen mustard were reduced after retrobulbar an- mechanical or chemical irritation by miosis, vasodila- aesthesia and abolished after postganglionic division tation and a breakdown of the blood-aqueous barrier. -

The Effect of Topical Application of 0.15% Ganciclovir Gel on Cytomegalovirus Corneal Endotheliitis
Clinical science The effect of topical application of 0.15% ganciclovir gel on cytomegalovirus corneal endotheliitis Noriko Koizumi,1,2 Dai Miyazaki,3 Tomoyuki Inoue,4 Fumie Ohtani,3 Michiko Kandori-Inoue,3 Tsutomu Inatomi,1 Chie Sotozono,1 Hiroko Nakagawa,1 Tomoko Horikiri,1 Mayumi Ueta,5 Takahiro Nakamura,5 Yoshitsugu Inoue,3 Yuichi Ohashi,4 Shigeru Kinoshita5 ▸ Additional material is ABSTRACT immunocompetent individuals suffering from anter- published online only. To view Background/aims The aim of this study was to ior uveitis with ocular hypertension45and corneal please visit the journal online – evaluate the therapeutic efficacy and drug transfer of endotheliitis.368 Clinical evidence of CMV-related (http://dx.doi.org/10.1136/ 6–11 bjophthalmol-2015-308238). topical application of 0.15% ganciclovir (GCV) gel on endotheliitis has accumulated, and current 1 cytomegalovirus (CMV) corneal endotheliitis. emphasis is on the importance of early diagnosis Department of 12–14 Ophthalmology, Kyoto Methods This study is a multicentre, prospective, and treatment. Prefectural University of interventional case series. Seven eyes of seven Several studies, including ours, have reported the Medicine, Kyoto, Japan immunocompetent patients diagnosed with CMV corneal usefulness of the systemic and topical application 2 Department of Biomedical endotheliitis, based on clinical manifestations and of anti-CMV medications, such as GCV and valgan- Engineering, Faculty of Life and 36810–14 Medical Sciences, Doshisha qualitative PCR, were enrolled in this study. The patients ciclovir. Our recent study of 106 eyes University, Kyoto, Japan were treated with topical applications of 0.15% GCV gel with CMV endotheliitis in Japan involved the sys- 3Division of Ophthalmology six times daily for 12 weeks without concomitant temic administration of anti-CMV drugs in 67.9% and Visual Science, Faculty of systemic GCV. -
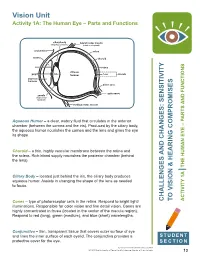
Vision Unit Activity 1A: the Human Eye – Parts and Functions
Vision Unit Activity 1A: The Human Eye – Parts and Functions ciliary body lateral rectus muscle (ring-shaped muscle) (rotation of eyeball) conjunctiva sclera cornea choroid iris retina vitreous pupil lens humour fovea macula aqueous humour blind spot optic nerve zonula (suspensory ligament) medical rectus muscle Aqueous Humor – a clear, watery fluid that circulates in the anterior chamber (between the cornea and the iris). Produced by the ciliary body, the aqueous humor nourishes the cornea and the lens and gives the eye its shape. Choroid – a thin, highly vascular membrane between the retina and the sclera. Rich blood supply nourishes the posterior chamber (behind the lens). Ciliary Body – located just behind the iris, the ciliary body produces aqueous humor. Assists in changing the shape of the lens as needed to focus. Cones – type of photoreceptor cells in the retina. Respond to bright light/ AND CHANGES: SENSITIVITY CHALLENGES VISION & HEARING COMPROMISES TO AND FUNCTIONS THE HUMAN EYE – PARTS 1A ACTIVITY illuminations. Responsible for color vision and fine detail vision. Cones are highly concentrated in fovea (located in the center of the macula region). Respond to red (long), green (medium), and blue (short) wavelengths. Conjunctiva – thin, transparent tissue that covers outer surface of eye and lines the inner surface of each eyelid. The conjunctiva provides a STUDENT protective cover for the eye. SECTION Teacher Enrichment Initiatives/CAINE 2013 © The University of Texas Health Science Center at San Antonio 13 Eyelid – Moveable fold of skin over the eye with lashes and glands along its margin. Eyelids provide protection from the environment, injury, and light. -

Postmortem Analyses of Vitreous Fluid
From the Department of Oncology-Pathology Karolinska Institutet, Stockholm, Sweden POSTMORTEM ANALYSES OF VITREOUS FLUID Brita Zilg Stockholm 2015 All previously published papers were reproduced with permission from the publisher. Published by Karolinska Institutet. Cover picture: Anatomy of the eye, by Ibn al-Haytham, ~1000 A.D. Printed by AJ E-Print 2015. © Brita Zilg, 2015 ISBN 978-91-7676-104-5 POSTMORTEM ANALYSES OF VITREOUS FLUID THESIS FOR DOCTORAL DEGREE (Ph.D.) By Brita Zilg Principal Supervisor: Opponent: Prof. Henrik Druid Prof. Burkhard Madea Karolinska Institutet University of Bonn Department of Oncology-Pathology Institute of Forensic Medicine Co-supervisor: Examination Board: Assoc. prof. Sören Berg Assoc. prof. Anders Ottosson University of Linköping University of Lund Department of Medicine and Health Department of Clinical Sciences Assoc. prof. Erik Edston University of Linköping Department of Medicine and Health Assoc. prof. Bo-Michael Bellander Karolinska Institutet Department of Clinical Neuroscience Institutionen för Onkologi-Patologi Postmortem Analyses of Vitreous Fluid AKADEMISK AVHANDLING som för avläggande av medicine doktorsexamen vid Karolinska Institutet offentligen försvaras i föreläsningssalen Rockefeller Fredagen den 6 november 2015, kl 09.00 av Brita Zilg Principal Supervisor: Opponent: Prof. Henrik Druid Prof. Burkhard Madea Karolinska Institutet University of Bonn Department of Oncology-Pathology Institute of Forensic Medicine Co-supervisor: Examination Board: Assoc. prof. Sören Berg Assoc. prof. Anders Ottosson University of Linköping University of Lund Department of Medicine and Health Department of Clinical Sciences Assoc. prof. Erik Edston University of Linköping Department of Medicine and Health Assoc. prof. Bo-Michael Bellander Karolinska Institutet Department of Clinical Neuroscience ABSTRACT The identification of various various medical conditions postmortem is often difficult.