Influence of Distance Between Boat Noise and Nest Site on the Behavior of Paternal Smallmouth Bass
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Appendix a Public Stakeholders List
Appendix A Public Stakeholders List © Hatch 2011/04 List of Landowners Goldie Clemenhagen Todd Wright 307 Little Rideau Lake Rd. RR 1 Portland, ON K0G 1V0 Portland, ON K0G 1V0 Terrence Wright Anne Gillespie 373 Little Rideau Lake Rd. 71 Metropole PVT Portland, ON K0G 1V0 Ottawa, ON K1Z 1E7 Propane MacDonald's William Brus PO Box 23 249 Little Rideau Lake Rd. Newboro, ON K0G 1P0 Portland, ON K0G 1V0 Ceri Lovell Ronald Bresee 248 Little Rideau Lake Rd. 54 McCann Rd. Portland, ON K0G 1V0 Portland, ON K0G 1V0 Eric Stoness Joan Flegg PO Box 59 4457 Bittersweet PL Westport, ON K0G 1X0 Gloucester, ON K1V 1R9 Reginald Scully Pierre Poulin 422 Little Rideau Lake Rd. Unit 206 434 McCann Rd. Portland, ON K0G 1V0 Portland, ON K0G 1V0 William Armstrong Brus William John Martin PO Box 488 316 L Rideau Lake Rd. Winchester, ON K0C 2K0 Portland, ON K0G 1V0 Homeowner Harry and Linda Barker 360 Narrows Lock Rd. 711 Narrows Locks Rd. Portland, ON K0G 1V0 Portland, ON K0G 1V0 List of Public and Government Agencies Sheldon Laidman, Manager of Development Services, Lesley Todd, Clerk, United Counties of Leeds and Grenville Township of Rideau Lakes 25 Central Avenue West, Suite 100 1439 County Road 8 Brockville, Ontario K6V 4N6 Delta, On K0E 1G0 Sandy Hay, County Planner, Works, Planning Services and Ministry of the Environment Asset Management, United Counties of Leeds and Grenville P.O. Box 22032 25 Central Avenue West, Suite 100 Kingston, Ontario K7M 8S5 Brockville, Ontario K6V 4N6 Trevor Dagilis, District Manager, Kingston District Office 1259 Gardiners Road Property Owner: Name Address (Including PO Box) City Province Postal Code Goldie Clemenhagen 307 Little Rideau Lake Rd. -

Ca. 200 Years) of FOUR LAKES WITHIN the RIDEAU CANAL SYSTEM, ONTARIO
RECONSTRUCTING THE TROPHIC HISTORIES (ca. 200 years) OF FOUR LAKES WITHIN THE RIDEAU CANAL SYSTEM, ONTARIO by Francine Forrest A thesis submitted to the Department of Biology in conformity with the requirements for the degree of Master of Science Queen's University Kingston. Ontario. Canada Apd. 2001 copyrightG Francine Forrest. ZOO 1 National Library Bibliothèque nationale l*i of Canada du Canada Acquisitions and Acquisitions et Bibliographic Services services bibliographiques 395 WeUington Street 395, nie Wellington Ottawa ON K1A ON4 Ottawa ON K1A ON4 Canada Canada The author has granted a non- L'auteur a accordé une licence non exclusive licence allowing the exclusive permettant à la National Library of Canada to Bibliothèque nationale du Canada de reproduce, loan, disûibute or seil reproduire, prêter, distribuer ou copies of this thesis in microfom, vendre des copies de cette thèse sous paper or electronic formats. la fome de microfiche/film, de reproduction sur papier ou sur format électronique. The author retains ownership of the L'auteur conserve la propriété du copyxight in this thesis. Neither the droit d'auteur qui protège cette thèse. thesis nor substantial extracts fiom it Ni la thèse ni des extraits substantiels may be printed or othenvise de celle-ci ne doivent être imprimés reproduced without the author' s ou autrement reproduits sans son permission. autorisation. ABSTRACT Diatom-based paleolimnological techniques were used to track the eutrophication histones (ca. 200 years) of four lakes within the Rideau Canal system. Ontario. Canada. The Rideau Canal watenvay links Kingston and Ottawa and was constructed in 1832 for rnilitary purposes. Recent water quality concems. -

MINUTES LAND DIVISION COMMITTEE the Land Division Committee Met in Regular Session on Monday, June 27, 2011 at 9:00 A.M. At
MINUTES LAND DIVISION COMMITTEE The Land Division Committee met in regular session on Monday, June 27, 2011 at 9:00 a.m. at the Lanark County Administration Building, 99 Christie Lake Road, Perth, Ontario. Members Present: R. Strachan, D. Murphy and W. Guthrie Staff Present: M. Kirkham, Secretary-Treasurer LAND DIVISION COMMITTEE Chair: R. Strachan 1. CALL TO ORDER A quorum was present. 2. DISCLOSURE OF PECUNIARY INTEREST None. 3. APPROVAL OF MINUTES MOTION #LD-2011-018 MOVED BY: SECONDED BY: “THAT, the minutes of the Land Division Committee meeting held on June 6, 2011 be approved as circulated.” ADOPTED 4. ADDITIONS & APPROVAL OF AGENDA MOTION #LD-2011-019 MOVED BY: SECONDED BY: “THAT, the agenda be adopted as circulated.” ADOPTED 5. DELEGATIONS & PRESENTATIONS None. 6. COMMUNICATIONS None 7. REPORTS 7.1 New Applications to be Heard. The Land Division Committee reviewed the reports for the following new applications to be considered at the 10:00 a.m. public hearings: 7.1.1 B10/ B10/153 – Paul S Taggart – New Lot & R-O-W Pt Lot 3 Conc. 5, geographic Township of North Burgess, now in Tay Valley Township. White Arrow Drive. 7.1.2 B10/163 & B10/164 – Muriel M Taggart, James E Taggart & Christopher B Taggart – two new lots & R-O-W Pt Lot 4/5 Conc. 5, geographic Township of North Burgess, now in Tay Valley Township. Yellow Arrow Drive. 7.1.3 B11/043 – Claire Larocque – lot addition Pt Lot 17 Conc. 12, Township of Beckwith. Patty Lane. 7.1.4 B11/048, B11/049 & B11/050 – John & Karen Miller – 3 new lots Pt Lot 11/12 Conc. -

Big Rideau Lake Association (BRLA) Annual General Meeting: DRAFT Minutes July 15Th, 2017 Portland Town Hall, Portland, Ontario 9:30 AM – 12:00 PM
Big Rideau Lake Association (BRLA) Annual General Meeting: DRAFT Minutes July 15th, 2017 Portland Town Hall, Portland, Ontario 9:30 AM – 12:00 PM Attendance: Brian Hawkins: President/Treasurer Rod Howes, Vice President Mary Sue Evans: Secretary Doug Kirkland: Government Relations Buzz Boles: Environmental Committee John Callan: Membership Committee Bill Belanger: Director at Large Toby Spry: Lake Safety Committee Regrets: Lyse Prendergast: Communications Guests: Brenda Howes Gayle Mathe Grant Leslie Phil Albert 1. Call to Order Brian Hawkins called the meeting to order at 9:34 a.m. There were 47 in attendance. 2. Welcome & Introduction (Moment of Silence) Brian Hawkins welcomed and thanked everyone for their attendance. As in tradition of the BRLA, a moment was taken to reflect on the past year and to give thanks for the joys, experiences and the loss of those on the lake. 3. Keynote Speaker Dr. Steven Cooke Buzz Boles introduced Dr. Steven Cooke. Dr. Cooke is a Canada Research Chair & Professor, Fish Ecology & Conservation Physiology Lab at Carleton University. Big Rideau Lake Association (BRLA) Minutes of Annual General Meeting: July 15, 2018 Minutes Taken By: Mary Sue Evans Page 1 of 11 Dr. Cooke thanked everyone for sharing the lake with his group. Dr. Cooke’s lab is focused on wild fish in the field. • Broad interests in all aspects of aquatic ecology, conservation biology, physiological ecology, fish behavior and environmental science • Research focused on understanding how individuals and populations respond to natural and anthropogenic stressors mostly in the field • Moving from problems to solutions Dr. Cooke’s lab is studying the ecology of black bass in the Big Rideau Lake. -

2013 Upper Rideau Lake Walleye FWIN Report Final
2013 Upper Rideau Lake Modified Fall Walleye Index Netting (FWIN) Report August 2015 Ontario Ministry of Natural Resources and Forestry (OMNRF) Kemptville District 1 Cette publication hautement spécialisée {2013 Upper Rideau Lake Modified Fall Walleye Index Netting (FWIN) Report} n'est disponible qu'en anglais conformément au Règlement 671/92, selon lequel il n’est pas obligatoire de la traduire en vertu de la Loi sur les services en français. Pour obtenir des renseignements en français, veuillez communiquer avec le ministère des Richesses naturelles et des Forêts au {613-258-8214 ou [email protected]}. 2013 Upper Rideau Lake Modified Fall Walleye Index Netting Assessment Report - OMNRF Kemptville District Page 2 TABLE OF CONTENTS Executive Summary 4 List of Figures 8 List of Tables 9 1.0 INTRODUCTION 10 2.0 METHODOLOGY 12 2.1 Sampling Methods 12 2.2 Sample Size 13 2.3 Gear Description 13 2.4 Biological Sampling 13 3.0 RESULTS & DISCUSSION 14 3.1 Total Catch - Fish Community 14 3.2 Walleye 16 3.3 Yellow Perch 24 3.4 Northern Pike 32 3.5 Other Fish Species 40 4.0 CONCLUSION & RECOMMENDATIONS 59 4.1 Conclusion 59 4.2 Recommendations 62 REFERENCES 63 APPENDIX A - Set and Lift Data for 2013 Upper Rideau Lake 64 Report Author: Joffre Cote Field Assessment Team: Eric Robertson, Joffre Cote, Shaun Thompson, Allen Bibby, Megan Reaney, Mary Dillon Cover Photo: Upper Rideau Lake Walleye Sampling, Rideau Lakes Twp, Leeds County (Photo Credit: Eric Robertson, MNRF) 2013 Upper Rideau Lake Modified Fall Walleye Index Netting Assessment Report - OMNRF Kemptville District Page 3 EXECUTIVE SUMMARY Upper Rideau Lake is a shallow meso-eutrophic lake that supports a diversity of mainly warm water and cool water fish communities. -

Figure 2-1: Mississippi-Rideau Source Protection Region
WHITEWATER REGION NORTH ALGONA WILBERFORCE KILMLALOEi, HsAGAsRTY iANsD RIsCHARiDpS pi-Rideau ± Clarence Creek Source Protection Region HORTON ¤£17 (! Fitzroy Habour (! Québec Ottawa River East QUEBEC Arnprior (! Ottawa River ADMASTON/BROMLEY Galetta Georgian Bay !( ARNPRIOR (! Dunrobin (! Ottawa (! !( MCNAB/BRAESIDE Lake Huron 417 ONTARIO Mississippi - Kinburn ¤£ C Rideau SPR (! a rp R iv MADAWASKA VALLEY er ¤£17 Marathon Ottawa River (! Carp Vars BRUDENELL, LYNDTOoronCto H AND RAGLAN COUNTY OF RENFREW (! (! !( Pakenham Lake Ontario ! ( Ottawa River OTTAWA Rochester r !( West Lower Mississippi Carp River e v i Buffalo U.S.A. !( R u UNITED COUNTIES a !( 0 60 120 240 e CITY OF OTTAWA d GREATER MADAWASKA i Lake Erie OF PRESCOTT R Kilometres Greely AND RUSSELL r (! ive R ck Jo RUSSELL Almonte Manotick !( MISSISSIPPI MILLS Metcalfe (! (! (! Richmond Appleton (! (! Indian River Clayton Lake Munster Ashton (! (! Kars (! Taylor Lake CARLETON PLACE Jock River Osgoode (!Carleton Place North Gower (! Dwyer Hill (! COUNTY OF LANARK (! UNITED COUNTIES OF STORMONT, DUNDAS LANARK HIGHLANDS Clyde River BECKWITH AND GLENGARRY COUNTY OF LENNOX NORTH FRONTENAC Lower Rideau AND ADDINGTON ¤£416 r e iv NORTH DUNDAS R Mississippi Lake i Lanark p r p ve Palmerston Lake i Ri (! s u Kemptville ADDINGTON HIGHLANDS is ea s d (! s i Buckshot Lake i R M Bedell CP Dam DRUMMOND/ (! Mazinaw Buckshot Creek NORTH ELMSLEY Burritts Rapids ! (! (Plevna Dalhousie Lake McDonalds Corner NORTH GRENVILLE (! Balderson (!Oxford Mills MONTAGUE High Falls (! k Upper Mazinaw Lake e -

Outlaw Fishing in Missouri
VOL 33 NO 4 APRIL 2008 Fisheries News Legislative Update Fisheries Journal Highlights FisheriesAmerican Fisheries Society • www.fisheries.org Calendar Job Center Outlaw Fishing in Missouri The Challenge of Managing Nearshore Rocky Reef Resources The North Atlantic Ocean: Fisheries • v o l 33 n o 4 The• a p r i l 2008 •Need w w w .f i s h e r i e s .foro r g Proactive Management157 Discovering the Painted Crayfish Photos courtesy of Ashley Frisch. Northwest Marine Technology, Inc. Painted crayfish Panulirus versicolor (above) are than one crayfish, the male can attract females to share widely exploited throughout the coral reefs of the Indo- his den. Ashley’s work also revealed that males with Pacific region, including Australia’s Great Barrier Reef. the largest dens can attract more than one female and They command a high price but relatively little is increase their reproductive potential. Males with dens known about their biology and population dynamics. large enough to attract females must fastidiously defend them from other male crayfish, about one third of the Ashley Frisch, at James Cook University, (photo lower population, that don’t have dens large enough to share right) is beginning to unlock some of the painted with a female. These “bachelor” males constantly roam crayfish’s secrets. His studies first required a technique the reef searching for a better den. to identify individuals. Ashley tested NMT’s injectable Visible Implant Elastomer tags and found them to be NMT is delighted to advise on projects and to help set highly suitable(1) (photo top right). -

Ontario FMZ Results
2015 Survey of Recreational Fishing in Canada: Selected Results for Fisheries Management Zones in Ontario This technical report should be cited as follows: Ontario Ministry of Natural Resources and Forestry. 2020. 2015 Survey of Recreational Fishing in Canada: Results for Fisheries Management Zones of Ontario. Fish and Wildlife Policy Branch. Ontario Ministry of Natural Resources and Forestry. Peterborough, Ontario. 61pp. Printed in Ontario, Canada MNRF Print: 978-1-4868-4729-7 PDF: 978-1-4868-4730-3 This publication was produced by: Fisheries Policy Section Fish and Wildlife Policy Branch Ontario Ministry of Natural Resources and Forestry 300 Water Street, Peterborough, Ontario 9J8M5 Cover photo courtesy of A. Skinner, 2020 This specialized publication, 2015 Survey of Recreational Fishing in Canada: Results for Fisheries Management Zones of Ontario is available in English only according to Regulation 411/97 which exempts it from translation under the French Language Services Act. To obtain information in French, please contact the Ministry of Natural Resources and Forestry at [email protected]. Cette publication hautement spécialisée, 2015 Survey of Recreational Fishing in Canada: Results for Fisheries Management Zones of Ontario n'est disponible qu'en anglais en vertu du Règlement 671/92 qui en exempte l'application de la Loi sur les services en français. Pour obtenir de l'aide en français, veuillez communiquer avec le ministère des richesses naturelles au [email protected]. 2015 Survey of Recreational Fishing in Canada: Ontario Results i Executive Summary The 2015 Survey of Recreational Fishing in Canada collected information from anglers about their recreational fishing activities to assess the economic and social importance of recreational fisheries to Canada’s provinces and territories. -

Palaeo-Indian and Archaic Occupations of the Rideau Lakes
WATSON: PALAEO-INDIAN AND ARCHAIC OCCUPATIONS 5 Palaeo-Indian and Archaic Occupations of the Rideau Lakes Gordon D. Watson Present knowledge of the Palaeo-Indian and Archaic in Ontario (Wright 1972; Kennedy 1966, 1970), occupations of the Rideau Lakes area is reported and New York (Ritchie 1969) and Ohio (Converse assessed. Recent adjustments to the dates when the 1973). The displays were updated in 1983 to Champlain Sea receded from eastern Ontario permit a present newer information based on Rideau Lakes reassessment of a side-notched fluted point from an area previously thought to have been flooded throughout surveys and excavations and on data from New Palaeo-Indian times. The identification of a lanceolate York (Funk 1976) and elsewhere in the Northeast point which is also side-notched suggests that these two (Trigger 1978). points may represent the beginning of the side-notching Archaeological work has included two field technique in late Palaeo-Indian times. seasons of survey and eight of excavation, under- Excavated evidence and radiocarbon dates from the taken to find new sites, to evaluate the potential of Wyght site (BfGa-11) confirm the presence of an early sites identified from the McLaren collection, and to Archaic component dating to 6000 B.C. on the eastern build a data base to aid in the classification and shoreline of Lower Rideau Lake. evaluation of the large surface collections from the Surface-collected Archaic projectile points of the area (Watson 1976b, 1977, 1979, 1980a, 1980b, Rideau Lakes have been classified by computer dis- 1981, 1982a, 1982b, 1983a, 1985). criminant analysis and the frequency of occurrence of different types is discussed. -

Evidence of Fish Spillover from Freshwater Protected Areas in Lakes of Eastern Ontario
Received: 19 March 2018 Revised: 1 April 2019 Accepted: 15 April 2019 DOI: 10.1002/aqc.3155 SPECIAL ISSUE ARTICLE Evidence of fish spillover from freshwater protected areas in lakes of eastern Ontario Aaron J. Zolderdo1,2 | Alice E.I. Abrams1 | Connor H. Reid1 | Cory D. Suski3 | Jon D. Midwood4 | Steven J. Cooke1 1 Department of Biology and Institute of Environmental Science, Carleton University, Abstract Ottawa, ON, Canada 1. Research has identified numerous conservation benefits attributed to the use of 2 Department of Biology, Queen's University, marine protected areas (MPAs), yet comparatively less is known about the effec- Kingston, ON, Canada tiveness of freshwater protected areas (FPAs). 3 Department of Natural Resources and Environmental Sciences, University of Illinois 2. This study assessed multiple long‐standing (>70 years active) intra‐lake FPAs in Urbana–Champaign, Urbana, IL three lakes in eastern Ontario, Canada, to evaluate their potential conservation 4 Great Lakes Laboratory for Fisheries and Aquatic Science, Fisheries and Oceans Canada, benefits. These FPAs were intended initially to protect exploited populations of Burlington, ON, Canada largemouth bass (Micropterus salmoides (Lacépède, 1802)), but since their estab- Correspondence lishment no empirical data have been collected to evaluate the effectiveness of Aaron J. Zolderdo, Department of Biology, FPAs for protecting bass or the broader fish community. Carleton University, 1125 Colonol By Dr., Ottawa, ON, Canada, K1S 5B6. 3. A comparative biological census of fish species abundance, biomass and species Email: [email protected] richness was conducted using snorkelling surveys within FPAs, along the border- Funding information ing transition zones, and in more distant non‐protected areas of the lake that Big Rideau Lake Association; Big Rideau Lake Environmental Fund; Canada Research Chairs; had similar habitats to the FPAs. -
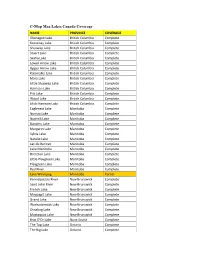
C-Map Max Lakes Canada Coverage
C-Map Max Lakes Canada Coverage NAME PROVINCE COVERAGE Okanagan Lake British Columbia Complete Kootenay Lake British Columbia Complete Shuswap Lake British Columbia Complete Stuart Lake British Columbia Complete Skaha Lake British Columbia Complete Lower Arrow Lake British Columbia Complete Upper Arrow Lake British Columbia Complete Kalamalka Lake British Columbia Complete Mara Lake British Columbia Complete Little Shuswap Lake British Columbia Complete Harrison Lake British Columbia Complete Pitt Lake British Columbia Complete Wood Lake British Columbia Complete Little Harrison Lake British Columbia Complete Eaglenest Lake Manitoba Complete Numao Lake Manitoba Complete Nutimik Lake Manitoba Complete Dorothy Lake Manitoba Complete Margaret Lake Manitoba Complete Sylvia Lake Manitoba Complete Natalie Lake Manitoba Complete Lac du Bonnet Manitoba Complete Lake Manitoba Manitoba Complete Brereton Lake Manitoba Complete Little Playgreen Lake Manitoba Complete Playgreen Lake Manitoba Complete Red River Manitoba Complete Lake Winnipeg Manitoba Partial Kennebecasis River New Brunswick Complete Saint John River New Brunswick Complete French Lake New Brunswick Complete Maquapit Lake New Brunswick Complete Grand Lake New Brunswick Complete Washademoak Lake New Brunswick Complete Otnabog Lake New Brunswick Complete Mactaquac Lake New Brunswick Complete Bras D'Or Lake Nova Scotia Complete The Top Lake Ontario Complete The Big Lake Ontario Complete Lake Muskoka Ontario Complete Mirror Lake Ontario Complete Lake Rosseau Ontario Complete Lake Joseph -

Survey of the Fish Assemblages of St. Lawrence Islands National Park in 2005
Canadian Manuscript Report of Fisheries and Aquatic Sciences 2777 2006 SURVEY OF THE FISH ASSEMBLAGES OF ST. LAWRENCE ISLANDS NATIONAL PARK IN 2005 by N.E. Mandrak, J. Barnucz and D. Marson Great Lakes Laboratory for Fisheries and Aquatic Sciences Department of Fisheries and Oceans Canada P.O. Box 5050, 867 Lakeshore Road Burlington, Ontario L7R 4A6 © Her Majesty the Queen in Right of Canada, 2006. Cat. No. Fs 97-4/2777E ISSN 0706-6473 Correct citation for this publication: Mandrak, N.E., J. Barnucz, G.J. Velema, and D. Marson. 2006. Survey of the fish assemblages of St. Lawrence Islands National Park in 2005. Can. Manuscr. Rep. Fish. Aquat. Sci. 2777: v + 17 pp. ii TABLE OF CONTENTS TABLE OF CONTENTS.......................................................................................III ABSTRACT.......................................................................................................... V RÉSUMÉ ............................................................................................................. V 1.0 INTRODUCTION........................................................................................1 2.0 METHODS .................................................................................................1 2.1 SITE SELECTION ..................................................................................1 2.2 ELECTROFISHING SAMPLING.............................................................1 2.3 FYKE NET SAMPLING...........................................................................5 2.4 MINNOW TRAP