New Collection Records for Theraphosidae (Araneae, Mygalomorphae) in Angola, with the Description of a Remarkable New Species of Ceratogyrus
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Nueva Especie De Citharacanthus Pocock, 1901 (Theraphosidae: Theraphosinae) Para México New Species of Citharacanthus Pocock, 1
Dugesiana 20(1): 63-66 Fecha de publicación: 30 de agosto de 2013 © Universidad de Guadalajara Nueva especie de Citharacanthus Pocock, 1901 (Theraphosidae: Theraphosinae) para México New species of Citharacanthus Pocock, 1901 (Theraphosidae: Theraphosinae) from Mexico Julio C. Estrada-Alvarez1, Cesar A. Guadarrama R.2 & Mónica Martínez O.3 1 Museo Universitario de Historia Natural “Dr. Manuel M. Villada”, Instituto Literario No. 100 Oriente, Colonia Centro, CP 50000, Toluca, Estado de México, [email protected], 2 Laboratorio de Investigación FUCESA, FUCESA.- Expertos en control de plagas-, Melchor Ocampo S/N, Col. Buenavista C.P.50200, Toluca, Edo. de México, México., 3Vivario del Zoológico Regional Miguel Álvarez de Toro. Calzada Cerro Hueco S/N Col. Zapotal CP. 29000 Tuxtla Gutiérrez, Chiapas. [email protected], [email protected]. gob.mx RESUMEN Se describe una especie nueva del género Citharacanthus Pocock, 1901 de Chiapas, México, esta especie se segregan de las restantes del género por la característica espermateca y receptáculos seminales. Palabras clave: Theraphosidae, Citharacanthus, México ABSTRACT A new species of the genus Citharacanthus Pocock, 1901 from Chiapas, Mexico is described and distinguishes from the congeners by the shape of spermathecae. Key words: Theraphosidae, Citharacanthus, Mexico INTRODUCCIÓN validas incluyendo a C. spinicrus (Latreille, 1819) y C. sargi En 1901 como resultado del desglose del antiguo genero (Strand, 1907). Eurypelma C. L. Koch, 1850, Pocock erige Citharacanthus con la El -

Malelane Safari Lodge, Kruger National Park
INVERTEBRATE SPECIALIST REPORT Prepared For: Malelane Safari Lodge, Kruger National Park Dalerwa Ventures for Wildlife cc P. O. Box 1424 Hoedspruit 1380 Fax: 086 212 6424 Cell (Elize) 074 834 1977 Cell (Ian): 084 722 1988 E-mail: [email protected] [email protected] Table of Contents 1. EXECUTIVE SUMMARY ............................................................................................................................ 3 2. INTRODUCTION ........................................................................................................................................... 5 2.1 DESCRIPTION OF PROPOSED PROJECT .................................................................................................................... 5 2.1.1 Safari Lodge Development .................................................................................................................... 5 2.1.2 Invertebrate Specialist Report ............................................................................................................... 5 2.2 TERMS OF REFERENCE ......................................................................................................................................... 6 2.3 DESCRIPTION OF SITE AND SURROUNDING ENVIRONMENT ......................................................................................... 8 3. BACKGROUND ............................................................................................................................................. 9 3.1 LEGISLATIVE FRAMEWORK .................................................................................................................................. -

Fasanbi SHOWCASE
Threatened Species Monitoring PROGRAMME Threatened Species in South Africa: A review of the South African National Biodiversity Institutes’ Threatened Species Programme: 2004–2009 Acronyms ADU – Animal Demography Unit ARC – Agricultural Research Council BASH – Big Atlassing Summer Holiday BIRP – Birds in Reserves Project BMP – Biodiversity Management Plan BMP-S – Biodiversity Management Plans for Species CFR – Cape Floristic Region CITES – Convention on International Trade in Endangered Species CoCT – City of Cape Town CREW – Custodians of Rare and Endangered Wildflowers CWAC – Co-ordinated Waterbird Counts DEA – Department of Environmental Affairs DeJaVU – December January Atlassing Vacation Unlimited EIA – Environmental Impact Assessment EMI – Environmental Management Inspector GBIF – Global Biodiversity Information Facility GIS – Geographic Information Systems IAIA – International Association for Impact Assessment IAIAsa – International Association for Impact Assessment South Africa IUCN – International Union for Conservation of Nature LAMP – Long Autumn Migration Project LepSoc – Lepidopterists’ Society of Africa MCM – Marine and Coastal Management MOA – memorandum of agreement MOU – memorandum of understanding NBI – National Botanical Institute NEMA – National Environmental Management Act NEMBA – National Environmental Management Biodiversity Act NGO – non-governmental organization NORAD – Norwegian Agency for Development Co–operation QDGS – quarter-degree grid square SABAP – Southern African Bird Atlas Project SABCA – Southern African -

Redescription of the Holotypes of Mygalarachnae Ausserer 1871 And
ARTÍCULO: Redescription of the holotypes of Mygalarachnae Ausserer 1871 and Harpaxictis Simon (1892) (Araneae: Theraphosidae) with rebuttal of their synonymy with Sericopelma Ausserer 1875. Ray Gabriel and Stuart.J. Longhorn ARTÍCULO: Abstract: Redescription of the holotypes of The examination of specimens from various Neotropical tarantula genera (The- Mygalarachnae Ausserer 1871 and raphosidae) indicated the unique nature of monotypic genera Mygalarachnae Harpaxictis Simon (1892) (Araneae: Ausserer 1871 and Harpaxictis Simon 1892. Review of the both holotypes leads Theraphosidae) with rebuttal of their us to argue that Mygalarachnae and Harpaxictis should be removed from their synonymy with Sericopelma current synonymy with Sericopelma Ausserer 1875. The presence of type I and Ausserer 1875. III urticating hairs on the holotype specimen of Mygalarachnae firmly place it in the subfamily Theraphosinae and we argue should be restored as a valid genus, Ray Gabriel so that current placement of “incertae sedis” is inappropriate. The identity of Hope Entomological Collections, Harpaxictis striatus is less certain, but here removed from synonymy with Seri- Oxford University Museum of Natural copelma, and due to a lack of other diagnostic features is suggested as nomen History, Parks Road, Oxford, dubium. Possible affinities of Mygalarachne with other valid genera of Thera- Oxon, England, OX1 3PW, UK. phosinae are briefly discussed. Key words: Sericopelma, Tarantula. Mygalarachne brevipes, Harpaxictis striatus, Stuart.J. Longhorn Taxonomy: Mygalarachne brevipes comb. rev., Harpaxictis striatus comb. rev. Dept. of Entomology. The Natural History Museum, Cromwell Road, London, SW7 5BD, Redescripción de los holotipos de Mygalarachnae Ausserer 1871 UK. y Harpaxictis Simon (1892) rechazando su sinonímia con Dept. of Biology. -

The Case of Embrik Strand (Arachnida: Araneae) 22-29 Arachnologische Mitteilungen / Arachnology Letters 59: 22-29 Karlsruhe, April 2020
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Arachnologische Mitteilungen Jahr/Year: 2020 Band/Volume: 59 Autor(en)/Author(s): Nentwig Wolfgang, Blick Theo, Gloor Daniel, Jäger Peter, Kropf Christian Artikel/Article: How to deal with destroyed type material? The case of Embrik Strand (Arachnida: Araneae) 22-29 Arachnologische Mitteilungen / Arachnology Letters 59: 22-29 Karlsruhe, April 2020 How to deal with destroyed type material? The case of Embrik Strand (Arachnida: Araneae) Wolfgang Nentwig, Theo Blick, Daniel Gloor, Peter Jäger & Christian Kropf doi: 10.30963/aramit5904 Abstract. When the museums of Lübeck, Stuttgart, Tübingen and partly of Wiesbaden were destroyed during World War II between 1942 and 1945, also all or parts of their type material were destroyed, among them types from spider species described by Embrik Strand bet- ween 1906 and 1917. He did not illustrate type material from 181 species and one subspecies and described them only in an insufficient manner. These species were never recollected during more than 110 years and no additional taxonomically relevant information was published in the arachnological literature. It is impossible to recognize them, so we declare these 181 species here as nomina dubia. Four of these species belong to monotypic genera, two of them to a ditypic genus described by Strand in the context of the mentioned species descriptions. Consequently, without including valid species, the five genera Carteroniella Strand, 1907, Eurypelmella Strand, 1907, Theumella Strand, 1906, Thianella Strand, 1907 and Tmeticides Strand, 1907 are here also declared as nomina dubia. Palystes modificus minor Strand, 1906 is a junior synonym of P. -

Molecular Basis of the Remarkable Species Selectivity of an Insecticidal
www.nature.com/scientificreports OPEN Molecular basis of the remarkable species selectivity of an insecticidal sodium channel toxin from the Received: 03 February 2016 Accepted: 20 June 2016 African spider Augacephalus Published: 07 July 2016 ezendami Volker Herzig1,*, Maria Ikonomopoulou1,*,†, Jennifer J. Smith1,*, Sławomir Dziemborowicz2, John Gilchrist3, Lucia Kuhn-Nentwig4, Fernanda Oliveira Rezende5, Luciano Andrade Moreira5, Graham M. Nicholson2, Frank Bosmans3 & Glenn F. King1 The inexorable decline in the armament of registered chemical insecticides has stimulated research into environmentally-friendly alternatives. Insecticidal spider-venom peptides are promising candidates for bioinsecticide development but it is challenging to find peptides that are specific for targeted pests. In the present study, we isolated an insecticidal peptide (Ae1a) from venom of the African spider Augacephalus ezendami (family Theraphosidae). Injection of Ae1a into sheep blowflies (Lucilia cuprina) induced rapid but reversible paralysis. In striking contrast, Ae1a was lethal to closely related fruit flies (Drosophila melanogaster) but induced no adverse effects in the recalcitrant lepidopteran pest Helicoverpa armigera. Electrophysiological experiments revealed that Ae1a potently inhibits the voltage-gated sodium channel BgNaV1 from the German cockroach Blattella germanica by shifting the threshold for channel activation to more depolarized potentials. In contrast, Ae1a failed to significantly affect sodium currents in dorsal unpaired median neurons from the American cockroachPeriplaneta americana. We show that Ae1a interacts with the domain II voltage sensor and that sensitivity to the toxin is conferred by natural sequence variations in the S1–S2 loop of domain II. The phyletic specificity of Ae1a provides crucial information for development of sodium channel insecticides that target key insect pests without harming beneficial species. -

Project Reports 2006
KNP 15/1/2 - 06 Project Reports 2006 Scientific Reports on Research Projects undertaken in the Kruger National Park during 2006 TABLE OF CONTENTS FELINE LENTIVIRUS: MOLECULAR ANALYSIS AND EPIDEMIOLOGY IN SOUTHERN AFRICAN LIONS ................................................................................. 13 Adams H .....................................................................................................................13 WILDLIFE CONSERVATION THROUGH PEOPLE CENTRED APPROACHES TO NATURAL RESOURCE MANAGEMENT AND THE CONTROL OF WILDLIFE EXPLOITATION........................................................................................................ 14 Algotsson EM ..............................................................................................................14 A REGIONAL SCALE PASSIVE MONITORING STUDY OF SULPHUR DIOXIDE (SO2), NITROGEN OXIDES (NOX) AND OZONE (O3) ........................................................ 15 Annegarn HJ ...............................................................................................................15 METAL ANALYSIS AND PHYSICO-CHEMICAL CHARACTERISTICS OF FOUR MAJOR RIVER SYSTEMS THAT TRANSECT THE KRUGER NATIONAL PARK (SOUTH AFRICA)..................................................................................................... 16 Barker HJ ....................................................................................................................16 TOWARDS A SOCIO-ECOLOGICAL SYSTEMS VIEW OF THE SAND RIVER CATCHMENT, SOUTH AFRICA: A RESILIENCE ANALYSIS -

Tarantulas and Social Spiders
Tarantulas and Social Spiders: A Tale of Sex and Silk by Jonathan Bull BSc (Hons) MSc ICL Thesis Presented to the Institute of Biology of The University of Nottingham in Partial Fulfilment of the Requirements for the Degree of Doctor of Philosophy The University of Nottingham May 2012 DEDICATION To my parents… …because they both said to dedicate it to the other… I dedicate it to both ii ACKNOWLEDGEMENTS First and foremost I would like to thank my supervisor Dr Sara Goodacre for her guidance and support. I am also hugely endebted to Dr Keith Spriggs who became my mentor in the field of RNA and without whom my understanding of the field would have been but a fraction of what it is now. Particular thanks go to Professor John Brookfield, an expert in the field of biological statistics and data retrieval. Likewise with Dr Susan Liddell for her proteomics assistance, a truly remarkable individual on par with Professor Brookfield in being able to simplify even the most complex techniques and analyses. Finally, I would really like to thank Janet Beccaloni for her time and resources at the Natural History Museum, London, permitting me access to the collections therein; ten years on and still a delight. Finally, amongst the greats, Alexander ‘Sasha’ Kondrashov… a true inspiration. I would also like to express my gratitude to those who, although may not have directly contributed, should not be forgotten due to their continued assistance and considerate nature: Dr Chris Wade (five straight hours of help was not uncommon!), Sue Buxton (direct to my bench creepy crawlies), Sheila Keeble (ventures and cleans where others dare not), Alice Young (read/checked my thesis and overcame her arachnophobia!) and all those in the Centre for Biomolecular Sciences. -
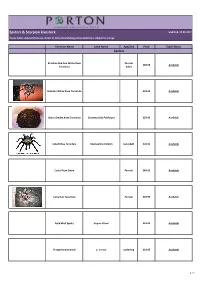
Spiders & Scorpion Livestock
Spiders & Scorpion Livestock Updated: 22.09.2017 Please Note: Livestock lists are correct at time of publishing and availability is subject to change Common Name Latin Name Age/Size Price Stock Status Spiders Brazilian Red And White Knee Female £49.95 Available Tarantula Adult Brazilian White Knee Tarantula £34.95 Available Chaco Golden Knee Tarantula Grammostola Pulchripes £29.95 Available Cobalt Blue Tarantula Haplopelma Lividum Sub Adult £49.95 Available Costa Rican Zebra Female £49.95 Available Curly Hair Tarantula Female £29.95 Available Field Wolf Spider Hogna Miami £19.95 Available Fringed Ornamental p. ornata spiderling £24.95 Available 1 / 4 Common Name Latin Name Age/Size Price Stock Status Spiders Golden Baboon Tarantula Augacephalus ezendami £39.95 Available Gooty Ornamental p. metallica spiderling £49.95 Available Green Bottle Blue Tarantula Female £49.95 Available Green Femur Birdeater Phormictopus"Green Femur" £39.95 Available Indian Ornamental Tarantula Poecilotheria regalis 2cm £24.95 Available King Baboon Tarantula Pelinobius Muticus £24.95 Available Martinique Pink Toe Tarantula 2cm £16.95 Available Mexican Fire Leg Tarantula Brachypelma Bohemi £39.95 Available Mexican Red Knee Tarantula Brachypelma Smithi £34.95 Available 2 / 4 Common Name Latin Name Age/Size Price Stock Status Spiders Mexican Red Leg Tarantula Brachypelma Emilia £39.95 Available £24.95 Available Mexican Red Rump Brachypelma Vagans Female Sub Adult Available £49.95 spiderling £19.95 Available OBT Tarantula Female Sub Adult Available £39.95 Purple Earth -

Arachnides 55
The electronic publication Arachnides - Bulletin de Terrariophile et de Recherche N°55 (2008) has been archived at http://publikationen.ub.uni-frankfurt.de/ (repository of University Library Frankfurt, Germany). Please include its persistent identifier urn:nbn:de:hebis:30:3-371590 whenever you cite this electronic publication. ARACHNIDES BULLETIN DE TERRARIOPHILIE ET DE RECHERCHES DE L’A.P.C.I. (Association Pour la Connaissance des Invertébrés) 55 DECEMBRE 2008 ISSN 1148-9979 1 EDITORIAL Voici le second numéro d’Arachnides depuis sa reparution. Le numéro 54 a été bien reçu par les lecteurs, sa version électronique facilitant beaucoup sa diffusion (rapidité et gratuité !). Dans ce numéro 55, de nombreux articles informent sur de nouvelles espèces de Theraphosidae ainsi qu’un bilan des nouvelles espèces de scorpions pour l’année 2007. Les lecteurs qui auraient des articles à soumettre, peuvent nous les faire parvenir par courrier éléctronique ou à l’adresse de l’association : DUPRE, 26 rue Villebois Mareuil, 94190 Villeneuve St Geoges. Une version gratuite est donc disponible sur Internet sur simple demande par l’intermédiaire du courrier électronique : [email protected]. Les annonces de parution sont relayées sur divers sites d’Internet et dans la presse terrariophile. L’A.P.C.I. vous annonce également que la seconde exposition Natures Exotiques de Verrières-le-Buisson aura lieu les 20 et 21 juin 2009. Dès que nous aurons la liste des exposants, nous en ferons part dans un futur numéro. Gérard DUPRE. E X O N A T U R E S I Mygales Q Scorpions U Insectes E Reptiles S Plantes carnivores Cactus..... -

Arachnides 76
Arachnides, 2015, n°76 ARACHNIDES BULLETIN DE TERRARIOPHILIE ET DE RECHERCHES DE L’A.P.C.I. (Association Pour la Connaissance des Invertébrés) 76 2015 0 Arachnides, 2015, n°76 LES PREDATEURS DES SCORPIONS (ARACHNIDA : SCORPIONES) G. DUPRE Dans leur revue sur les prédateurs de scorpions, Polis, Sissom & Mac Cormick (1981) relèvent 150 espèces dont essentiellement des espèces adaptées au comportement nocturne de leur proie (chouettes, rongeurs, carnivores nocturnes) mais également des espèces diurnes (lézards, rongeurs, carnivores....) qui débusquent les scorpions sous les pierres ou dans leurs terriers. Dans une précédente note (Dupré, 2008) nous avions effectué un relevé afin d'actualiser cette étude de 1981. Sept ans après, de nouvelles données sont présentées dans cette synthèse. Voici un nouveau relevé des espèces prédatrices. Nous ne faisons pas mention des scorpions qui feront l'objet d'un futur article traité avec le cannibalisme. Explication des tableaux: La première colonne correspond aux prédateurs, la seconde aux régions concernées et la troisième aux références. Dans la mesure du possible, les noms scientifiques ont été rectifiés en fonction des synonymies ou des nouvelles combinaisons appliquées depuis les dates de publication d'origine. ARTHROPODA ARACHNIDA SOLIFUGAE Solifugae Afrique du Nord Millot & Vachon, 1949; Punzo, 1998; Cloudsley-Thompson, 1977 Eremobates sp. USA Bradley, 1983 ARACHNIDA ARANEAE Acanthoscurria atrox Brésil Lourenço, 1981 Aphonopelma sp. et autres Amérique centrale Mazzotti, 1964 Teraphosidae Phormictopus auratus Cuba Teruel & De Armas, 2012 Brachypelma vagans Mexique Dor et al., 2011 Epicadus heterogaster Brésil Lourenço et al. 2006 Latrodectus sp. USA Baerg, 1961 L. hesperus USA Polis et al., 1981 L. mactans Cuba Teruel, 1996; Teruel & De Armas, 2012 L. -

WO 2017/035099 Al 2 March 2017 (02.03.2017) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2017/035099 Al 2 March 2017 (02.03.2017) P O P C T (51) International Patent Classification: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, C07C 39/00 (2006.01) C07D 303/32 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, C07C 49/242 (2006.01) HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, (21) International Application Number: MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PCT/US20 16/048092 PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (22) International Filing Date: SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, 22 August 2016 (22.08.2016) TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (26) Publication Language: English GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, (30) Priority Data: TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, 62/208,662 22 August 2015 (22.08.2015) US TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, (71) Applicant: NEOZYME INTERNATIONAL, INC.